Quest for the right Drug
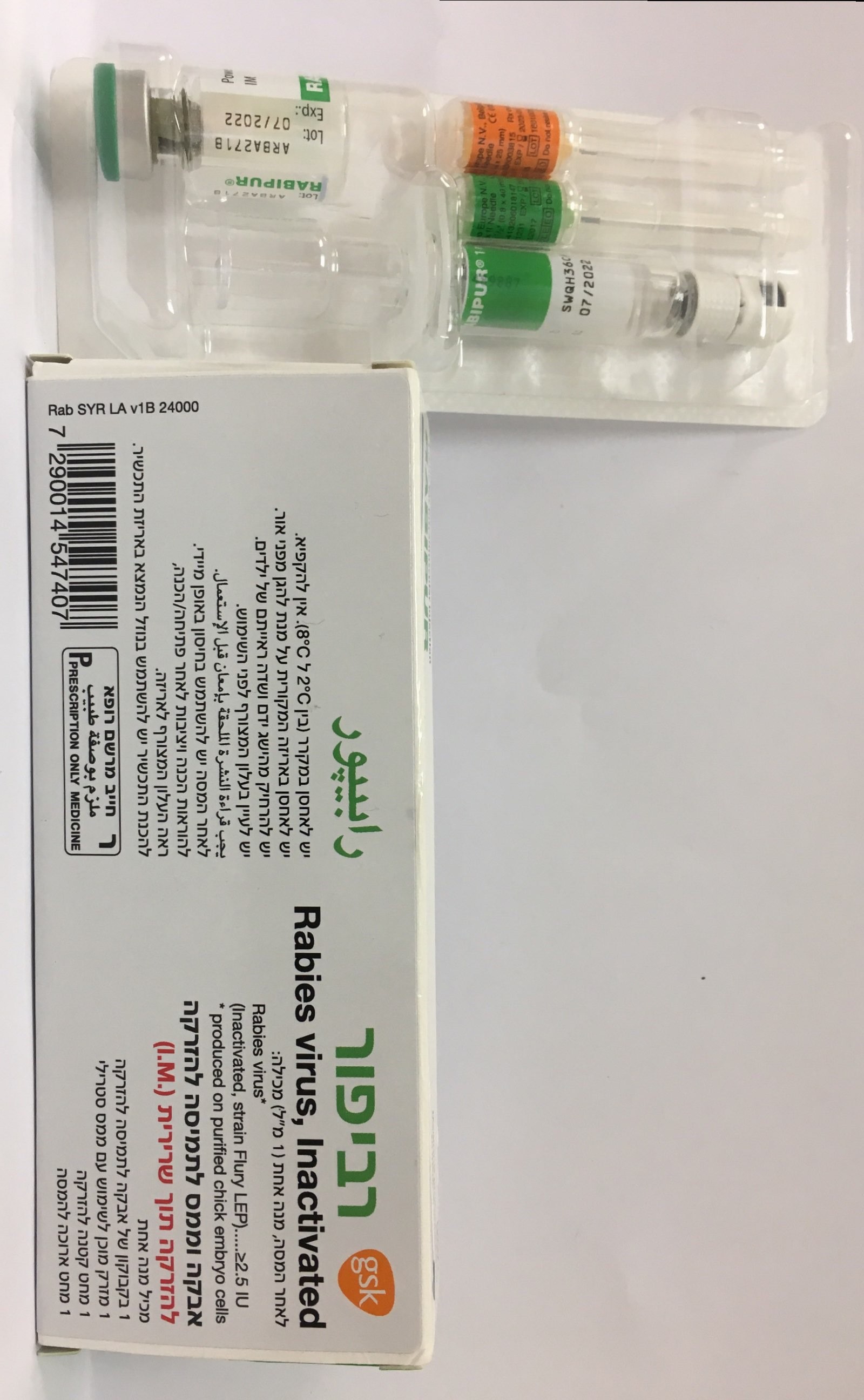
רביפור RABIPUR (RABIES, INACTIVATED, WHOLE VIRUS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
אבקה וממס להכנת תמיסה להזרקה : POWDER AND SOLVENT FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile Anaphylactic reactions including anaphylactic shock that are very rare but clinically severe, and potentially lethal, systemic allergic reactions, can occur following Rabipur vaccination. Mild allergic reactions to Rabipur (i.e. hypersensitivity), including rashes (very common) and urticaria (common) may occur after vaccination. These reactions are usually mild in nature and typically resolve within a few days. Very rare cases with symptoms of Encephalitis and Guillain-Barré Syndrome have been reported following Rabipur vaccination. In clinical trials, the most commonly reported solicited adverse reactions were injection site pain (30-85%) or injection site induration (15-35%). Most injection site reactions were not severe and resolved within 24 to 48 hours. Tabulated list of adverse reactions Adverse reactions considered as being at least possibly related to vaccination have been categorised by frequency. Frequencies are defined as follows: Rabipur_Vial_PFS_SPC_V4.1_Update_09-2019 Page 6 of 12 Very common: (≥1/10) Common: (≥1/100 to <1/10) Uncommon: (≥1/1,000 to <1/100) Rare: (≥1/10,000 to <1/1,000) Very rare: (<1/10,000) Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness. In addition to reports in clinical trials, worldwide voluntary reports of adverse reactions received for Rabipur since market introduction are included in the list. These reactions are reported voluntarily from a population of uncertain size and have been chosen for inclusion due to their seriousness, frequency of reporting, causal relationship to Rabipur, or a combination of these factors. Table 5: Adverse reactions reported in clinical trials and in postmarketing surveillance System Organ Class Frequency Adverse events Blood and lymphatic system Common Lymphadenopathy disorders Immune system disorders Rare Hypersensitivity Very rare Anaphylaxis including anaphylactic shock* Metabolism and nutrition Common Decreased appetite disorder Nervous system disorders Very common Headache, Dizziness Rare Paraesthesia Very rare Encephalitis*, Guillain-Barré syndrome*, Presyncope*, Syncope*, Vertigo* Gastrointestinal disorders Common Nausea, Vomiting, Diarrhoea, Abdominal pain/ discomfort Skin and subcutaneous tissue Very common Rash disorders Common Urticaria Rare Hyperhidrosis (sweating) Very rare Angioedema* Musculoskeletal and Common Myalgia, Arthralgia connective tissue disorders General disorder and Very common Injection site reactions, Malaise, administration site conditions Fatigue, Asthenia, Fever Rare Chills *Additional adverse reactions from spontaneous reporting Paediatric population Frequency, type and severity of adverse reactions in children are expected to be the same as in adults. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Rabipur_Vial_PFS_SPC_V4.1_Update_09-2019 Page 7 of 12 Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/. Additionally, you should also report to GSK Israel (il.safety@gsk.com).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
