Quest for the right Drug
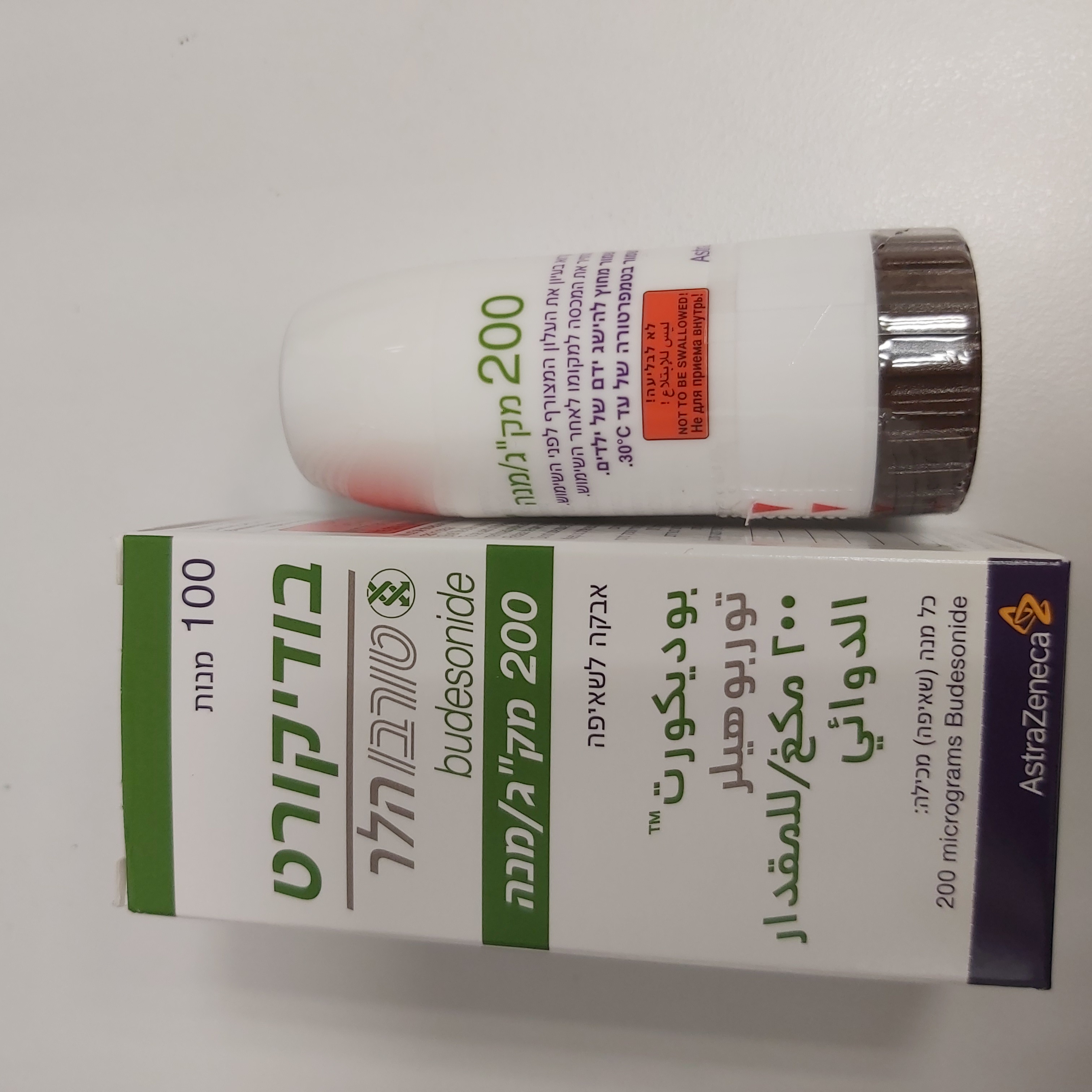
בודיקורט טורבוהלר 200 מק"ג/מנה BUDICORT TURBUHALER 200 MCG/DOSE (BUDESONIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
שאיפה : INHALATION
צורת מינון:
אבקה לשאיפה : POWDER FOR INHALATION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Special caution is necessary in patients with active or quiescent pulmonary tuberculosis, and in patients with fungal or viral infections in the airways. Non steroid-dependent patients: A therapeutic effect is usually reached within 10 days. In patients with excessive mucus secretion in the bronchi, a short (about 2 weeks) additional oral corticosteroid regimen can be given initially. Steroid-dependent patients: When transferral from oral steroids to Budicort Turbohaler is started, the patient should be in a relatively stable phase. A high dose of Budicort Turbohaler is then given in combination with the previously used oral steroid dose for about 10 days. After that, the oral steroid dose should be gradually reduced (by for example 2.5 milligrams prednisolone or the equivalent each month) to the lowest possible level. In many cases, it is possible to completely substitute Budicort for the oral steroid. During transfer from oral therapy to Budicort, a generally lower systemic steroid action will be experienced which may result in the appearance of allergic or arthritic symptoms such as rhinitis, eczema and muscle and joint pain. Specific treatment should be initiated for these conditions. During the withdrawal of oral steroids, patients may feel unwell in a non-specific way, even though respiratory function is maintained or improved. Patients should be encouraged to continue with Budicort therapy whilst withdrawing the oral steroid, unless there are clinical signs to indicate the contrary. . A general insufficient glucocorticosteroid effect should be suspected if, in rare cases, symptoms such as tiredness, headache, nausea and vomiting should occur. In these cases a temporary increase in the dose of oral glucocorticosteroids is sometimes necessary. As with other inhalation therapy, paradoxical bronchospasm may occur, with an immediate increase in wheezing after dosing. If this occurs, treatment with inhaled budesonide should be discontinued immediately, the patient assessed and alternative therapy instituted if necessary. Patients who have previously been dependent on oral steroids may, as a result of prolonged systemic steroid therapy, experience the effects of impaired adrenal function. Recovery may take a considerable amount of time after cessation of oral steroid therapy, hence oral steroid-dependent patients transferred to budesonide may remain at risk from impaired adrenal function for some considerable time. In such circumstances, HPA axis functions should be monitored regularly. Acute exacerbations of asthma may need an increase in the dose of Budicort or additional treatment with a short course of oral corticosteroid and/or an antibiotic, if there is an infection. The patient should be advised to use a shortacting inhaled bronchodilator as rescue medication to relieve acute asthma symptoms. Budicort is not intended for rapid relief of acute episodes of asthma where an inhaled short-acting bronchodilator is required. If patients find short-acting bronchodilator treatment ineffective or they need more inhalations than usual, medical attention must be sought. In this situation consideration should be given to the need for or an increase in their regular therapy, e.g. higher doses of inhaled budesonide or the addition of a long-acting beta agonist, or for a course of oral glucocorticosteroid. Patients, who have required high dose emergency corticosteroid therapy or prolonged treatment at the highest recommended dose of inhaled corticosteroids, may also be at risk of impaired adrenal function. These patients may exhibit signs and symptoms of adrenal insufficiency when exposed to severe stress. Additional systemic corticosteroid treatment should be considered during periods of stress or elective surgery. These patients should be instructed to carry a steroid warning card indicating their needs. Treatment with supplementary systemic steroids or Budicort should not be stopped abruptly. Systemic effects may occur with any inhaled corticosteroids, particularly at high doses prescribed for long periods. These effects are much less likely to occur with inhalation treatment than with oral corticosteroids. Possible systemic effects include Cushing's syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, decrease in bone mineral density, cataract, glaucoma and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). It is important, therefore, that the dose of inhaled corticosteroid is titrated to the lowest dose at which effective control of asthma is maintained. Reduced liver function affects the elimination of corticosteroids causing lower elimination rate and higher systemic exposure. Be aware of possible systemic side effects. The plasma clearance following an intravenous dose of budesonide however was similar in cirrhotic patients and in healthy subjects. After oral ingestion systemic availability of budesonide was increased by compromised liver function due to decreased first pass metabolism. The clinical relevance of this to treatment with Budicort is unknown as no data exist for inhaled budesonide, but increases in plasma levels and hence an increased risk of systemic adverse effects could be expected. Co-treatment with CYP3A inhibitors, e.g. itraconazole, ketoconazole, HIV protease inhibitors and cobicistat- containing products is expected to increase the risk of systemic corticosteroid side effects. Therefore, the combination should be avoided unless the benefit outweighs this increased risk, in which case patients should be monitored for systemic corticosteroid side effects. This is of limited clinical importance for short-term (1-2 weeks) treatment with itraconazole or ketoconazole or other potent CYP3A inhibitors, but should be taken into consideration during long-term treatment. A reduction in the dose of budesonide should also be considered (see section 4.5). Oral candidiasis may occur during the therapy with inhaled corticosteroids. This infection may require treatment with appropriate antifungal therapy and in some patients discontinuation of treatment may be necessary (see section 4.2). Pneumonia in patients with COPD An increase in the incidence of pneumonia, including pneumonia requiring hospitalisation, has been observed in patients with COPD receiving inhaled corticosteroids. There is some evidence of an increased risk of pneumonia with increasing steroid dose but this has not been demonstrated conclusively across all studies. There is no conclusive clinical evidence for intra-class differences in the magnitude of the pneumonia risk among inhaled corticosteroid products. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of such infections overlap with the symptoms of COPD exacerbations. Risk factors for pneumonia in patients with COPD include current smoking, older age, low body mass index (BMI) and severe COPD. Visual disturbance Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids. Paediatric population Influence on growth It is recommended that the height of children receiving prolonged treatment with inhaled corticosteroids is regularly monitored. If growth is slowed, therapy should be re-evaluated with the aim of reducing the dose of inhaled corticosteroid, if possible, to the lowest dose at which effective control of asthma is maintained. The benefit of the corticosteroid therapy and the possible risk of growth suppression must be carefully weighed. In addition, consideration should be given to referring the patient to a paediatric respiratory specialist.
Effects on Driving
4.7 Effects on ability to drive and use machines Budicort Turbohaler has no or negligible influence on the ability to drive and use machines.

שימוש לפי פנקס קופ''ח כללית 1994
Bronchial asthma. יירשם ע"י רופא מומחה למחלות ריאה ואלרגולוג
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
