Quest for the right Drug
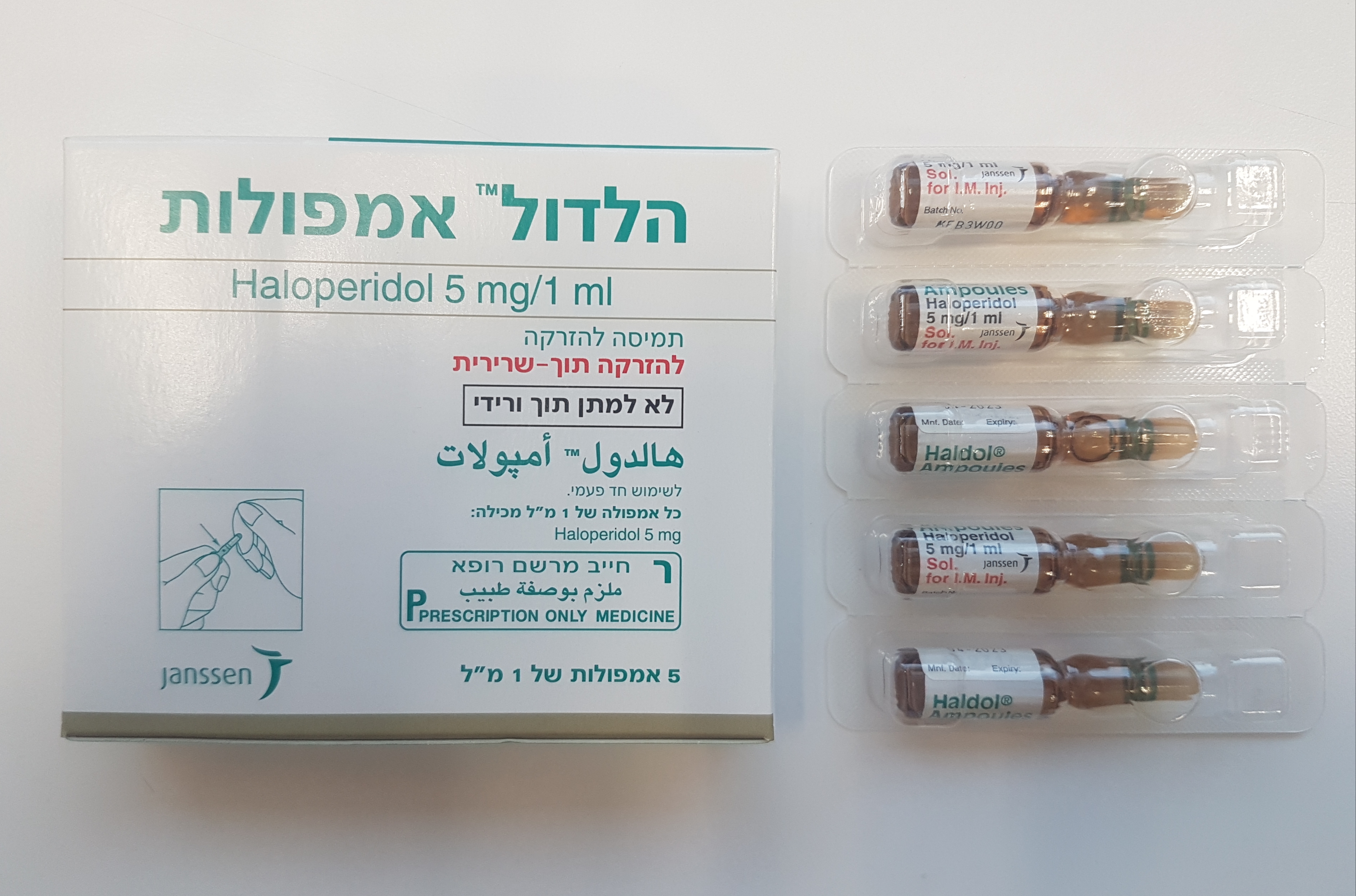
הלדול אמפולות HALDOL AMPOULES (HALOPERIDOL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1. Pharmacodynamic properties Pharmacotherapeutic group: psycholeptics; antipsychotics; butyrophenone derivatives, ATC code N05AD01 Mechanism of action Haloperidol is an antipsychotic, belonging to the butyrophenones group. It is a potent central dopamine type 2 receptor antagonist and at recommended doses, has low alpha-1 antiadrenergic activity and no antihistaminergic or anticholinergic activity. Pharmacodynamic effects Haloperidol suppresses delusions and hallucinations as a direct consequence of blocking dopaminergic signalling in the mesolimbic pathway. The central dopamine blocking effect has activity on the basal ganglia (nigrostriatal bundles). Haloperidol causes efficient psychomotor sedation, which explains the favourable effect on mania and other agitation syndromes. The activity on the basal ganglia probably underlies the undesirable extrapyramidal motor effects (dystonia, akathisia and parkinsonism). The antidopaminergic effects of haloperidol on lactotropes in the anterior pituitary explain hyperprolactinaemia due to inhibition of dopamine-mediated tonic inhibition of prolactin secretion. Additionally, the antidopaminergic effect on the chemoreceptor-trigger zone of the area postrema explains the activity against nausea and vomiting.
Pharmacokinetic Properties
5.2. Pharmacokinetic properties Absorption Following intramuscular administration, haloperidol is completely absorbed. Peak plasma concentrations of haloperidol are attained within 20 to 40 minutes. Distribution Mean haloperidol plasma protein binding in adults is approximately 88 to 92%. There is a high inter-subject variability for plasma protein binding. Haloperidol is rapidly distributed to various tissues and organs, as indicated by the large volume of distribution (mean values 8 to 21 l/kg after intravenous dosing). Haloperidol crosses the blood-brain barrier easily. It also crosses the placenta and is excreted in breast milk. Biotransformation Haloperidol is extensively metabolised in the liver. The main metabolic pathways of haloperidol in humans include glucuronidation, ketone reduction, oxidative N-dealkylation and formation of pyridinium metabolites. The metabolites of haloperidol are not considered to make a significant contribution to its activity; however, the reduction pathway accounts approximately for 23% of the biotransformation, and back-conversion of the reduced metabolite of haloperidol to haloperidol cannot be fully ruled out. The cytochrome P450 enzymes CYP3A4 and CYP2D6 are involved in haloperidol metabolism. Inhibition or induction of CYP3A4, or inhibition of CYP2D6, may affect haloperidol metabolism. A decrease in CYP2D6 enzyme activity may result in increased haloperidol concentrations. Elimination The terminal elimination half-life of haloperidol is on average 21 hours (range 13 to 36 hours) after intramuscular administration. Haloperidol apparent clearance after extravascular administration ranges from 0.9 to 1.5 l/h/kg and is reduced in poor metabolisers of CYP2D6. Reduced CYP2D6 enzyme activity may result in increased concentrations of haloperidol. The inter-subject variability (coefficient of variation, %) in haloperidol clearance was estimated to be 44% in a population pharmacokinetic analysis in patients with schizophrenia. After intravenous haloperidol administration, 21% of the dose was eliminated in the faeces and 33% in the urine. Less than 3% of the dose is excreted unchanged in the urine. Linearity/non-linearity A linear relationship exists between haloperidol dose and plasma concentrations in adults. Special populations Elderly Haloperidol plasma concentrations in elderly patients were higher than in younger adults administered the same dose. Results from small clinical studies suggest a lower clearance and a longer elimination half-life of haloperidol in elderly patients. The results are within the observed variability in haloperidol pharmacokinetics. Dose adjustment is recommended in elderly patients (see section 4.2). Renal impairment The influence of renal impairment on the pharmacokinetics of haloperidol has not been evaluated. About one-third of a haloperidol dose is excreted in urine, mostly as metabolites. Less than 3% of administered haloperidol is eliminated unchanged in the urine. Haloperidol metabolites are not considered to make a significant contribution to its activity, although for the reduced metabolite of haloperidol, back-conversion to haloperidol cannot be fully ruled out. Even though impairment of renal function is not expected to affect haloperidol elimination to a clinically relevant extent, caution is advised in patients with renal impairment, and especially those with severe impairment, due to the long half-life of haloperidol and its reduced metabolite, and the possibility of accumulation (see section 4.2). Because of the high haloperidol distribution volume and its high protein binding, only very small amounts are removed by dialysis. Hepatic impairment The influence of hepatic impairment on the pharmacokinetics of haloperidol has not been evaluated. However, hepatic impairment may have significant effects on the pharmacokinetics of haloperidol because it is extensively metabolised in the liver. Therefore, half the initial dose and caution is advised in patients with hepatic impairment (see sections 4.2 and 4.4). Pharmacokinetic/pharmacodynamics relationships Therapeutic concentrations Based on published data from multiple clinical studies, therapeutic response is obtained in most patients with acute or chronic schizophrenia at plasma concentrations of 1 to 10 ng/ml. A subset of patients may require higher concentrations as a consequence of a high inter-subject variability in haloperidol pharmacokinetics. In patients with first-episode schizophrenia, therapeutic response may be obtained at concentrations as low as 0.6 to 3.2 ng/ml, as estimated based on measurements of D2 receptor occupancy and assuming that a D2 receptor occupancy level of 60 to 80% is most appropriate for obtaining therapeutic response and limiting extrapyramidal symptoms. On average, concentrations in this range would be obtained with doses of 1 to 4 mg daily. Due to the high inter-subject variability in haloperidol pharmacokinetics and the concentration-effect relationship, it is recommended to adjust the individual haloperidol dose based on the patient’s response, taking into account data suggesting a lag time of 5 days to reach half of the maximal therapeutic response. Measurement of haloperidol blood concentrations may be considered in individual cases. Cardiovascular effects The risk of QTc prolongation increases with haloperidol dose and with haloperidol plasma concentrations. Extrapyramidal symptoms Extrapyramidal symptoms can occur within the therapeutic range, although the frequency is usually higher with doses producing higher than therapeutic concentrations.

שימוש לפי פנקס קופ''ח כללית 1994
Psychotic disorders, Gilles de la Tourette's syndrome, Huntington's chorea
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
