Quest for the right Drug
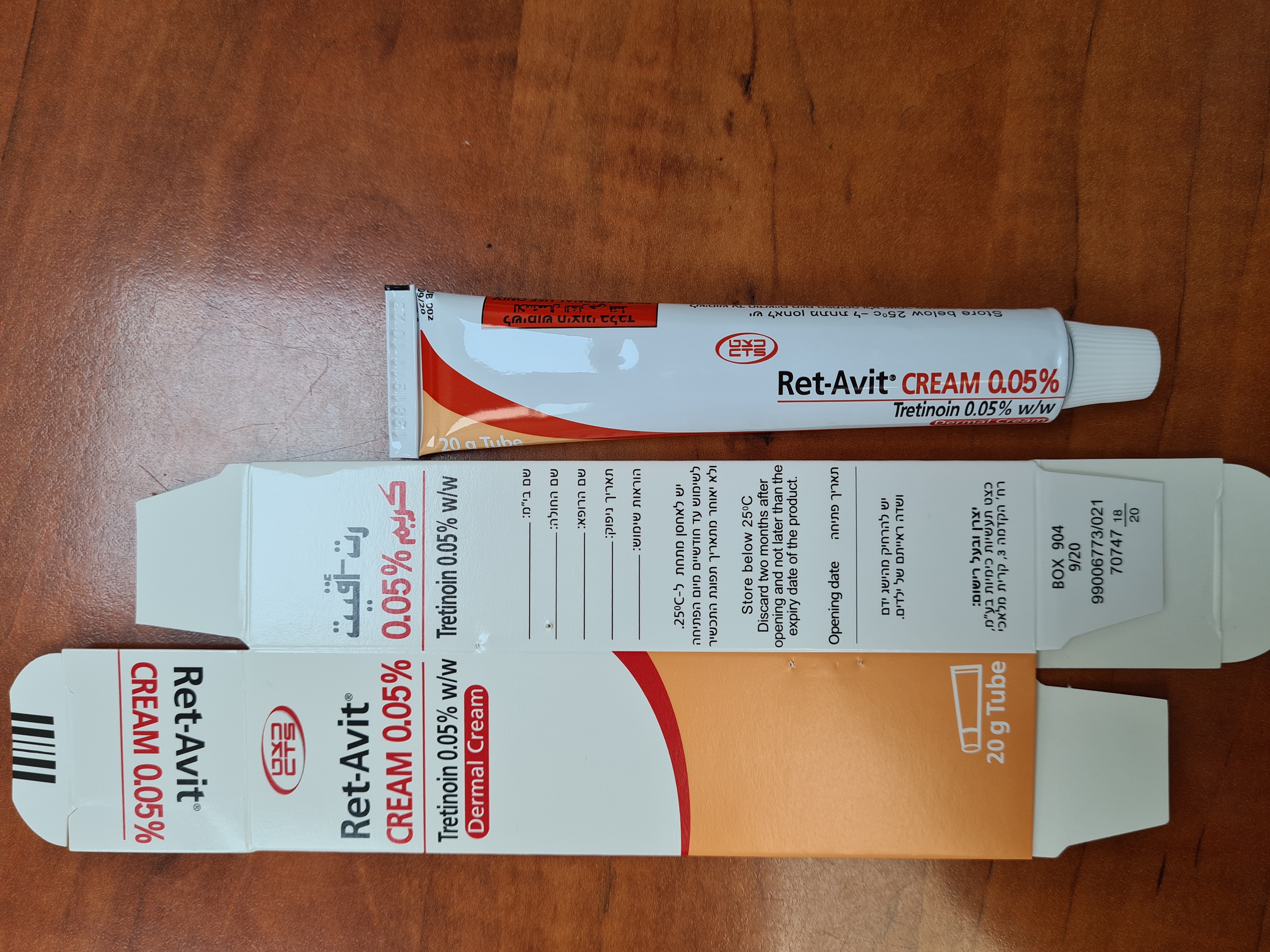
רט-אויט קרם % 0.05 RET-AVIT CREAM 0.05 % (TRETINOIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
עורי : DERMAL
צורת מינון:
קרם : CREAM
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Application of excessive amounts of product will not provide increased efficacy, but may increase the potential for irritation. Even at the recommended usage, the skin of certain individuals may become excessively dry, red, or swollen. If the degree of irritation warrants, patients should be directed to temporarily reduce the amount or frequency of application of the medication, discontinue use temporarily, or discontinue use altogether. Excessive skin dryness may also be experienced; if so, use of an appropriate moisturizer during the day may be helpful. Unprotected exposure to excessive sunlight or UV exposure, including sunlamps and solaria, should be minimized during the use of RET-AVIT. Patients with sunburn should be advised not to use the product on the affected areas until fully recovered because of heightened susceptibility to additional irritation to patients under treatment with tretinoin. Patients who may have considerable sun exposure due to occupation and those with inherent sensitivity to the sun should exercise particular caution. Use of sunscreen products and protective clothing over treated areas are recommended when exposure cannot be avoided. Weather extremes, such as wind or cold, also may be irritating to patients under treatment with tretinoin. RET-AVIT should be kept away from the mucous regions of the eyes, mouth, and nose. If contact with these areas occurs, wash carefully with water. There is evidence that, at least in some animal models, tretinoin may have photocarcinogenic potential, although some studies have suggested that tretinoin inhibits photocarcinogenesis. The relevance of this finding to use in man is uncertain. It is however advisable that patients avoid or minimise exposure to sunlight. Due to the high concentration of alcohol in RET-AVIT GEL, the product is flammable. Do not smoke a cigarette or be exposed to fire until the applied gel is completely dry.
Effects on Driving
4.7 Effects on ability to drive and use machines None known. The topical administration of RET-AVIT is not considered likely to affect the patient’s ability to drive or use machines.

שימוש לפי פנקס קופ''ח כללית 1994
Acne. יירשם ע"י רופא עור
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
