Quest for the right Drug
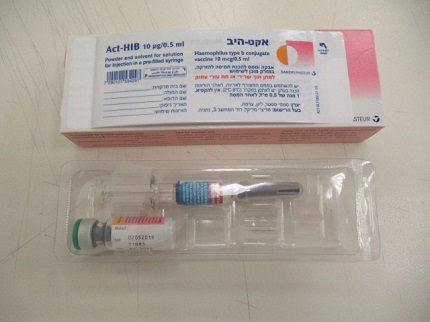
אקט-היב ACT-HIB (HAEMOPHILUS B)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי, תוך-שרירי : S.C, I.M
צורת מינון:
אבקה מיובשת בהקפאה להזרקה : LYOPHILIZED POWDER FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
5 WARNINGS AND PRECAUTIONS 5.1 Management of Acute Allergic Reactions Epinephrine and other appropriate agents must be available should an acute anaphylactic reaction occur. 5.2 Guillain-Barré Syndrome If Guillain-Barré syndrome has occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the decision to give any tetanus toxoid-containing vaccine, including ActHIB vaccine, should be based on careful consideration of the potential benefits and possible risks. 5.3 Altered Immunocompetence In immunosuppressed persons, including those receiving immunosuppressive therapy, the expected antibody responses may not be obtained. 5.4 Limitations of Vaccine Effectiveness Vaccination with ActHIB vaccine may not protect 100% of individuals. 5.5 Tetanus Immunization Immunization with ActHIB vaccine does not substitute for routine tetanus immunization. 5.6 Interference with Laboratory Tests Urine antigen detection may not have a diagnostic value in suspected disease due to H. influenzae type b within 1 to 2 weeks after receipt of a H. influenzae type b-containing vaccine, including ActHIB [see Drug Interactions (7.3)]. 6 ADVERSE REACTIONS 6.1 Clinical Trials Experience Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice. More than 7,000 infants and young children (≤2 years of age) have received at least one dose of ActHIB vaccine during US clinical trials. Of these, 1,064 subjects 12 to 24 months of age who received ActHIB vaccine alone reported no serious or life threatening adverse reactions. Adverse reactions associated with ActHIB vaccine generally subsided after 24 hours and did not persist beyond 48 hours after immunization. In a US trial, the safety of ActHIB vaccine was evaluated in 110 children 15 to 20 months of age. All children received three doses of Haemophilus influenzae type b conjugate vaccine (ActHIB vaccine or a previously licensed Haemophilus b conjugate vaccine) at approximately 2, 4, and 6 months of age. The incidence of selected solicited injection site and systemic adverse reactions which occurred within 48 hours following the dose of ActHIB vaccine is shown in Table 1. Table 1: Local and Systemic Reactions at 6, 24, and 48 Hours Following Immunization with ActHIB Vaccine in Children 15 to 20 months old Adverse Event 6 Hrs. Post-dose 24 Hrs. Post-dose 48 Hrs. Post-dose Local (%) N=110 N=110 N=110 Tenderness 20.0 8.2 0.9 Erythema 0.0 0.9 0.0 (>1") Induration* 5.5 3.6 0.9 Swelling 3.6 1.8 0.0 Systemic (%) N=103-110 N=105-110 N=104-110 Fever (>102.2°F) 0 1.0 1.9 (>39.0°C) Irritability 27.3 20.9 12.7 Drowsiness 36.4 17.3 12.7 Anorexia 12.7 10.0 6.4 Vomiting 0.9 0.9 0.9 Persistent cry 0 0 0 Unusual cry 0 0 0 * Induration is defined as hardness with or without swelling. In a US clinical trial (P3T06), 1,454 children were enrolled and received one dose of ActHIB vaccine at 2 months of age and subsequent doses administered at 4 and 6 months of age (concomitantly with DAPTACEL [a US-licensed diphtheria, tetanus and pertussis vaccine], IPOL [a US-licensed inactivated poliovirus vaccine] and PCV7 [Pneumococcal conjugate vaccine, 7-valent]) vaccines at 2, 4, and 6 months of age and hepatitis B vaccine at 2 and 6 months of age). At 15-16 months of age, 418 children received a 4 th dose of ActHIB and DAPTACEL vaccines. The most frequent systemic reactions following any dose (>50% of participants) were decreased activity/lethargy, fussiness/irritability, and inconsolable crying. Table 2: Number (Percentage) of Children with Selected Solicited Systemic Adverse Reactions by Severity Occurring within 0-3 days After Vaccination in Study P3T06 DAPTACEL + DAPTACEL + IPOL + ActHIB Vaccines ActHIB Vaccines Systemic Reactions Dose 1 Dose 2 Dose 3 Dose 4 N=1,390-1,406 N=1,346-1,360 N=1,301-1,312 N=379-381 % % % % Fever*† ≥38.0°C 9.3 16.1 15.8 8.7 >38.5°C 1.6 4.3 5.1 3.2 >39.5°C 0.1 0.4 0.3 0.8 Decreased Activity/Lethargy‡ Any 51.1 37.4 33.2 24.1 Moderate or Severe 24.3 15.8 12.7 9.2 Severe 1.2 1.4 0.6 0.3 Inconsolable Crying Any 58.5 51.4 47.9 36.2 ≥1 hour 16.4 16.0 12.2 10.5 >3 hours 2.2 3.4 1.4 1.8 Fussiness/Irritability Any 75.8 70.7 67.1 53.8 ≥1 hour 33.3 30.5 26.2 19.4 >3 hours 5.6 5.5 4.3 4.5 Note. - Ages of study participants ranged from 1.3 to 19.5 months. * Fever is based upon actual temperatures recorded with no adjustments to the measurement route. † Following Doses 1- 3 combined, the proportion of temperature measurements that were taken by axillary, rectal or other routes, or not recorded were 4 4 .8%, 54 .0%, 1.0%, and 0.1%, respectively. Following Dose 4 , the proportion of temperature measurements that were taken by axillary, rectal or other routes, or not recorded were 61.1%, 36.6%, 1.7%, and 0.5%, respectively. ‡ Moderate: interferes with or limits usual daily activity; Severe: disabling, not interested in usual daily activity. In Study P3T06, within 30 days following any of Doses 1-3 of DAPTACEL + IPOL + ActHIB vaccines, 50 of 1,455 (3.4%) participants experienced a serious adverse event (SAE). One SAE of seizure with apnea occurring on the day of vaccination with the first dose of the three vaccines was determined by the investigators as possibly related. Within 30 days following Dose 4, four of 418 (1.0%) participants who received DAPTACEL + ActHIB vaccines experienced a serious adverse event. None was assessed by the investigators as related to the study of vaccines. 6.2 Post marketing Experience The following events have been spontaneously reported during the post-approval use of ActHIB vaccine. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. Immune system disorders: Anaphylaxis, other allergic/hypersensitivity reactions (including urticaria, angioedema) Nervous system disorders: Convulsions General disorders and administration s ite conditions: Extensive limb swelling, peripheral edema, pruritus, rash (including generalized rash)
Effects on Driving

שימוש לפי פנקס קופ''ח כללית 1994
Immunization of children 2 months to 5 years of age against invasive disease caused by Haemophilus influenzae type b
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
