Quest for the right Drug
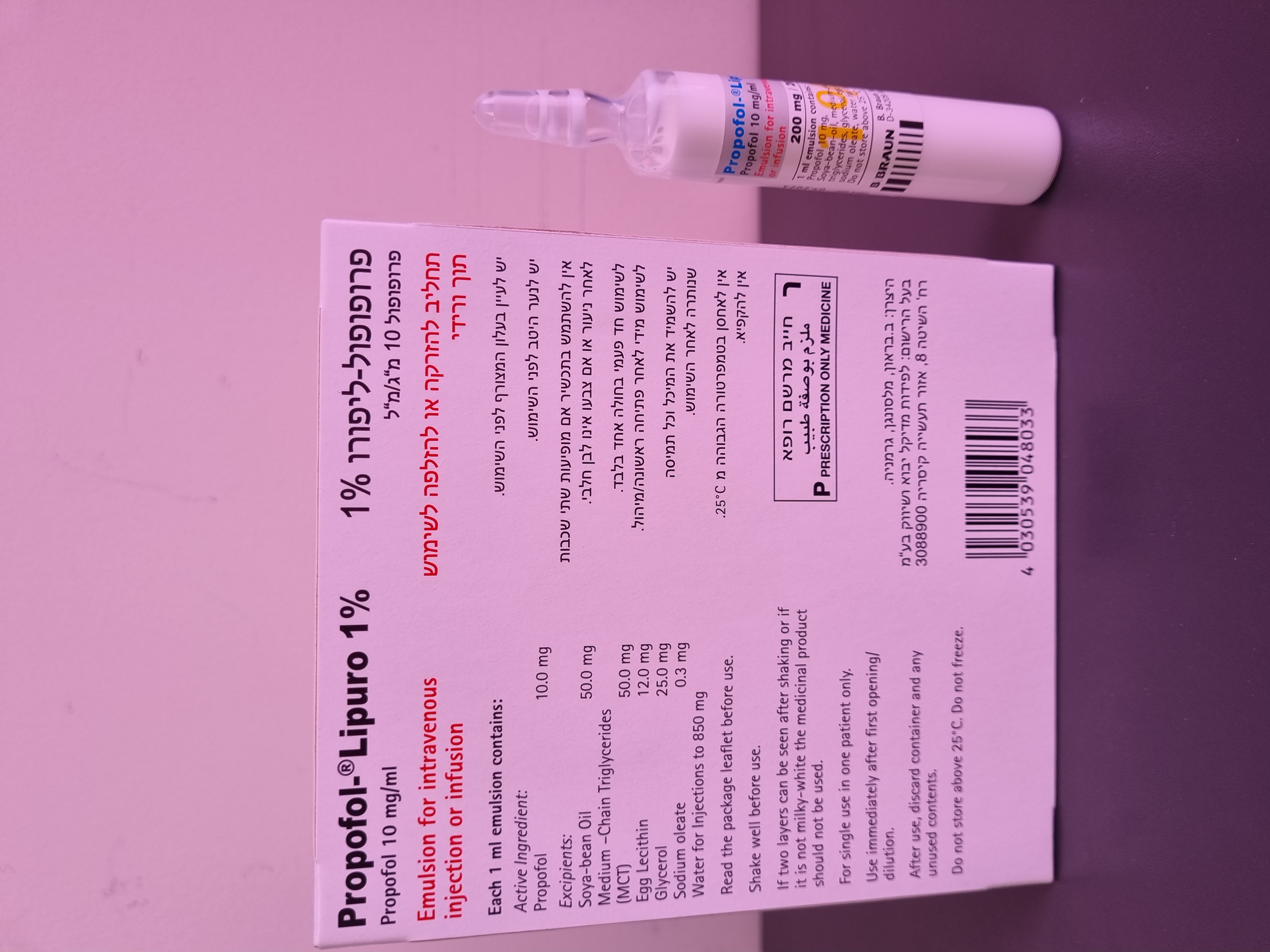
פרופופול ליפורו % 1 PROPOFOL - LIPURO 1 % (PROPOFOL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תחליב להזרקה או אינפוזיה : EMULSION FOR INJECTION OR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of Excipients Soya-bean oil, refined, medium-chain triglycerides, glycerol, egg lecithin, sodium oleate, water for injections. 6.2 Incompatibilities This medicinal product must not be mixed with other products except those mentioned in section 6.6. 6.3 Shelf Life 2 years. After first opening: to be used immediately. After dilution according to directions: aAdministration of dilution must commence immediately after preparation. 6.4 Special Precautions for Storage Do not store above 25 °C. Do not freeze. Keep the ampoules and vials in the outer carton in order to protect from light. 6.5 Nature and Contents of Container Colourless Type I glass ampoules containing 20 ml of emulsion. Colourless Type II glass vials sealed with bromobutyl rubber stoppers containing 50 ml or 100 ml of emulsion. Pack sizes: glass ampoules : 5 x 20 ml glass vials: 10 x 20 ml, 1 x 50 ml, 10 x 50 ml, 1 x 100 ml, 10 x 100 ml Not all pack sizes may be marketed. 6.6 Special precautions for disposal and other handlings Any unused product or waste material should be disposed of in accordance with local requirements. Containers should be shaken before use. For single use only. Any portion of contents remaining after use must be discarded, see section 4.2. If two layers can be seen after shaking or if it is not milky-white the medicinal product should not be used. Propofol-Lipuro 1% should only be mixed with the following products: glucose 50 mg/ml (5 % w/v) solution, sodium chloride 9 mg/ml (0.9% w/v) solution, or sodium chloride 1.8 mg/ml (0.18 %) and glucose 40 mg/ml (4 % w/v) solution, and preservative-free lidocaine injection 1% (see section 4.2 "Method and duration of administration" "Infusion of diluted Propofol-Lipuro 1%“) Co-administration of Propofol-Lipuro 1% together with glucose solution 50 mg/ml (5% w/v) solution or sodium chloride 9 mg/ml (0.9% w/v) solution, or sodium chloride 1.8 mg/ml (0.18 % w/v) and glu- cose 40 mg/ml (4 % w/v) solution via a Y-connector close to the injection site is possible. 7 Registration numbers: 127 87 30672 00 127 87 30672 11 8 Manufacturer: B.Braun Melsungen AG 34212 Melsungen Germany

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
