Quest for the right Drug
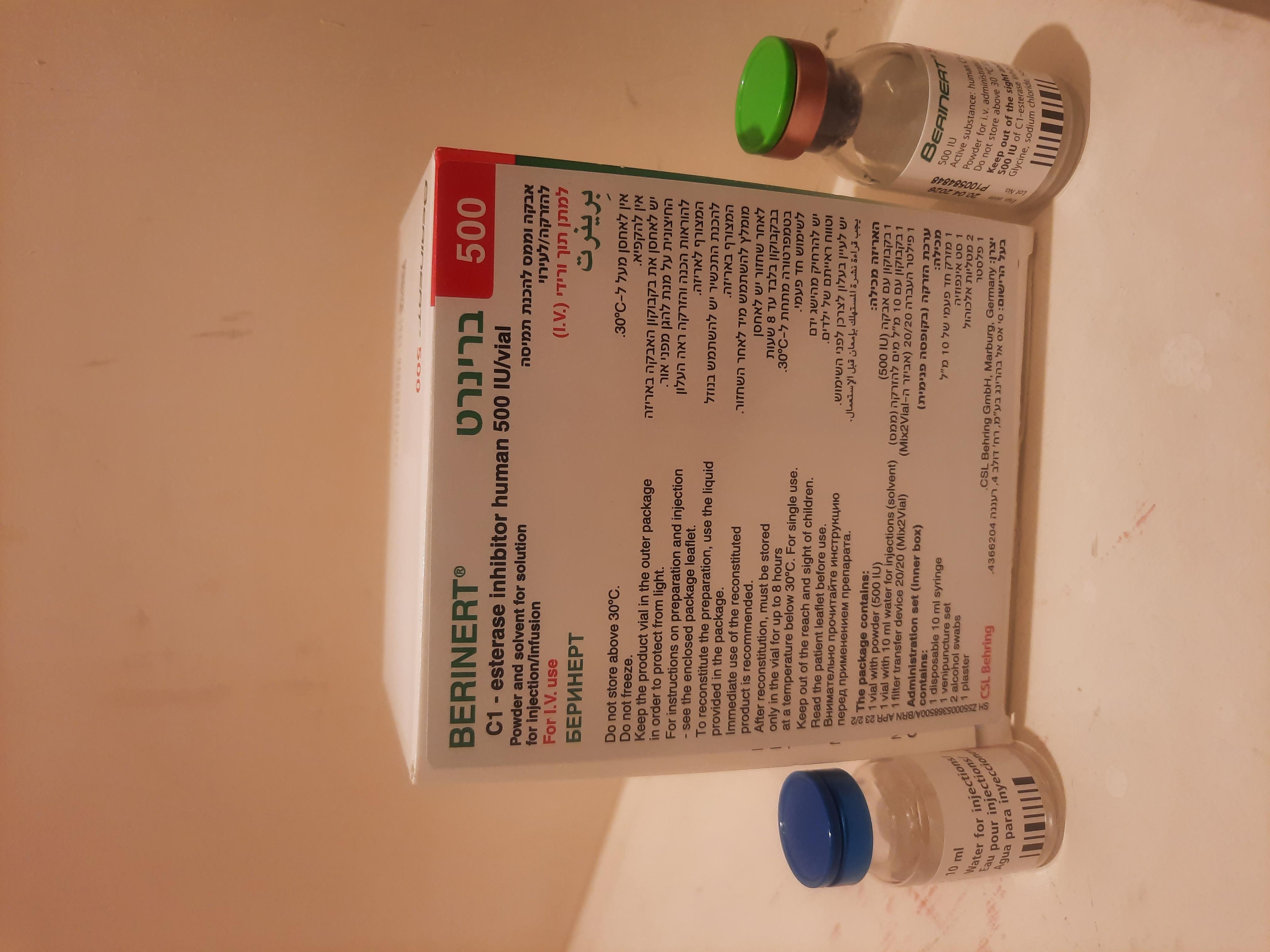
ברינרט BERINERT (C - 1 ESTERASE INHIBITOR HUMAN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להמסה להזרקהאינפוזיה : POWDER FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Powder: Glycine Sodium chloride Sodium citrate Solvent: Water for injections 6.2 Incompatibilities In the absence of compatibility studies this medicinal product must not be mixed with other medicinal products and diluents in the syringe/infusion set. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. After reconstitution, the physico-chemical stability of Berinert has been demonstrated for 48 hours at maximum 30 °C. From a microbiological point of view and as Berinert contains no preservative, the reconstituted product should be used immediately. If it is not administered immediately, storage shall not exceed 8 hours when stored below +30 0C. The reconstituted product should only be stored in the vial. 6.4 Special precautions for storage Do not store above 30 °C. Do not freeze. Keep the vial in the outer carton, in order to protect from light. For storage conditions after reconstitution of the medicinal product, see section 6.3. 6.5 Nature and contents of container Immediate containers : Powder (500 IU) in a vial (Type II glass) with a stopper (bromobutyl rubber), old gold seal (aluminium) and lime flip-off cap (plastic). 10 ml of solvent in a vial (Type I glass) with a stopper (chlorobutyl rubber), blue seal (aluminium) and blue flip-off cap (plastic). Presentation Box containing: 1 vial with powder 1 solvent vial (10 ml) 1 filter transfer device 20/20 (Mix2Vial) Administration set (inner box): 1 disposable 10 ml syringe 1 venipuncture set 2 alcohol swabs 1 plaster Pack size of 1. 6.6 Special precautions for disposal Any unused medicinal product or waste material should be disposed of in accordance with local requirements. Method of administration General instructions - The solution should be colourless and clear. - After filtering/withdrawal (see below) reconstituted product should be inspected visually for particulate matter and discoloration prior to administration. - Do not use solutions that are cloudy or have deposits. - Reconstitution and withdrawal must be carried out under aseptic conditions. Use the syringe provided with the product or a silicone-free syringe. Reconstitution Bring the solvent to room temperature. Ensure product and solvent vial flip caps are removed and the stoppers are treated with an antiseptic solution and allowed to dry prior to opening the Mix2Vial package. 1. Open the Mix2Vial package by peeling off the lid. Do not remove the Mix2Vial from the blister package! 1 2. Place the solvent vial on an even, clean surface and hold the vial tight. Take the Mix2Vial together with the blister package and push the spike of the blue adapter end straight down through the solvent vial 2 stopper. 3. Carefully remove the blister package from the Mix2Vial set by holding at the rim, and pulling vertically upwards. Make sure that you only pull away the blister package and not the Mix2Vial set. 3 4. Place the product vial on an even and firm surface. Invert the solvent vial with the Mix2Vial set attached and push the spike of the transparent adapter end straight down through the product vial stopper. The solvent will automatically flow into the product vial. 4 5. With one hand grasp the product-side of the Mix2Vial set and with the other hand grasp the solvent-side and unscrew the set carefully counterclockwise into two pieces. Discard the solvent vial with the blue Mix2Vial adapter attached. 5 6. Gently swirl the product vial with the transparent adapter attached until the substance is fully dissolved. Do not shake. 6 7. Draw air into an empty, sterile syringe. Use the syringe provided with the product or a silicone-free syringe. While the product vial is upright, connect the syringe to the Mix2Vial’s Luer Lock fitting by screwing clockwise. Inject air into the product vial. 7 Withdrawal and application 8. While keeping the syringe plunger pressed, turn the system upside down and draw the solution into the syringe by pulling the plunger back slowly. 8 9. Now that the solution has been transferred into the syringe, firmly hold on to the barrel of the syringe (keeping the syringe plunger facing down) and disconnect the transparent Mix2Vial adapter from the syringe by unscrewing counterclockwise. 9

פרטי מסגרת הכללה בסל
"התרופה תינתן לטיפול סימפטומטי בהתקפים חריפים של אנגיואדמה תורשתית בחולים עם חסר ב-C1 esterase inhibitor ובהתקיים כל אלה: 1. החולה מצוי בטיפול ומעקב של מרפאה לאימונולוגיה קלינית; 2. החולה סובל מהתקפים חוזרים של כאבי בטן חזקים או התקפים חוזרים של היצרות לרינקס; 3.הטיפול יינתן באישור מומחה באלרגיה ואימונולוגיה המטפל בחולה במסגרת מרפאה לאימונולוגיה קלינית; 4. לא יינתנו לחולה באותו התקף שתי התרופות – CONESTAT ALFA, ICATIBANT, C1 ESTERASE INHIBITOR, HUMAN.
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
10/01/2012
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
