Quest for the right Drug
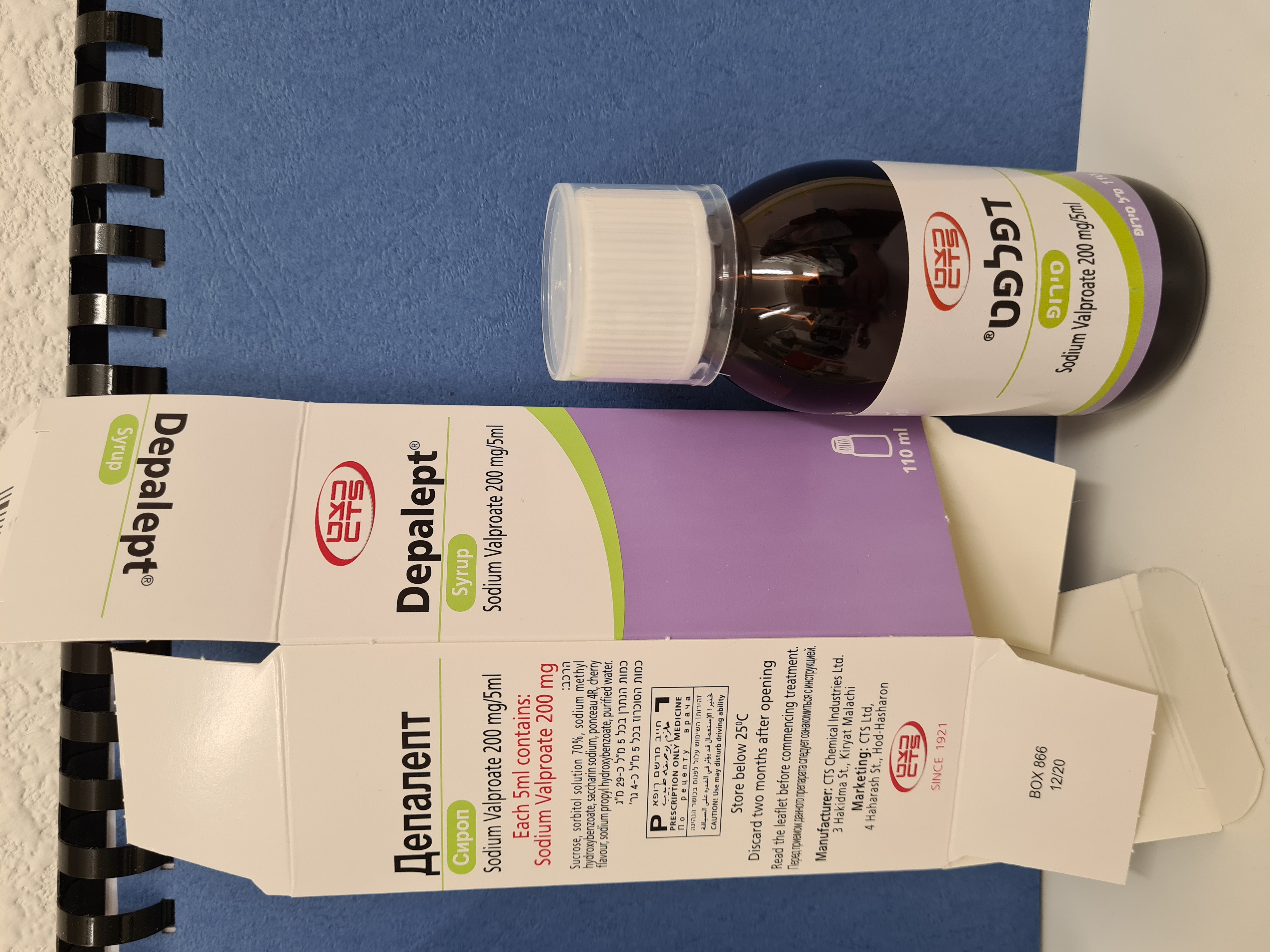
דפאלפט סירופ DEPALEPT SYRUP (VALPROIC ACID AS SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
סירופ : SYRUP
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Interactions : אינטראקציות
4.5. Interaction with other medicinal products and other forms of interaction Contraindicated combinations + St. John's Wort There is a risk of decreased plasma concentrations and reduced efficacy of the antiepileptic. Inadvisable combinations + Lamotrigine There is a higher risk of serious skin reactions (toxic epidermal necrolysis). Furthermore, an increase in lamotrigine plasma concentrations may occur (decreased hepatic metabolism by sodium valproate). If coadministration proves necessary, close clinical monitoring is required. + Penems (carbapenems) There is a risk of seizures due to a rapid decrease in valproic acid plasma concentrations, which may become undetectable. Co-administration of valproic acid and carbapenems has led to decreases in plasma concentrations of valproic acid of approximately 60 to 100% in around two days. Due to the rapid onset and the extent of the decreased plasma concentrations, simultaneous administration of carbapenems in patients stabilised on valproic acid who cannot be monitored should therefore be avoided (see section 4.4). Combinations requiring precautions for use + Acetazolamide Increased hyperammonemia with increased risk of encephalopathy may occur. Regular monitoring of clinical and laboratory parameters is required. + Aztreonam There is a risk of seizures due to a decrease in valproic acid plasma concentrations. Clinical monitoring, plasma assays and possible dose adjustment of the anticonvulsant are required during treatment with the anti-infective agent and after its discontinuation. + Carbamazepine Increased plasma concentrations of the active metabolite of carbamazepine with signs of overdose may occur. In addition, reduced valproic acid plasma concentrations may occur due to its increased hepatic metabolism by carbamazepine. Clinical monitoring, plasma assays and dose adjustment of both anticonvulsants are required. + Felbamate Increased serum valproic acid concentrations with a risk of overdose may occur. Clinical monitoring and monitoring of laboratory parameters and possible valproate dose adjustment are required during treatment with felbamate and after its discontinuation. + Estrogen-containing products, including estrogen-containing hormonal contraceptives Estrogens are inducers of the UDP-glucuronosyl transferase (UGT) isoforms involved in valproate glucuronidation and may increase valproate clearance, which in turn is thought to cause a decrease in serum valproate concentrations and to potentially reduce valproate efficacy (see section 4.4). Consider monitoring valproate serum levels. Conversely, valproate has no enzyme-inducing effect; as a consequence, valproate does not reduce the efficacy of estro-progestative agents in women receiving hormonal contraception. + Metamizole Metamizole may decrease valproate serum levels when co-administered, which may result in potentially decreased valproate clinical efficacy. Prescribers should monitor clinical response (seizure control or mood control) and consider monitoring valproate serum levels as appropriate. + Nimodipine (oral route and, by extrapolation, by injection) There is a risk of a 50% increase in plasma nimodipine concentrations. Therefore, nimodipine dose reduction is necessary in hypotensive patients. + Phenobarbital, and by extrapolation primidone Increased hyperammonemia with increased risk of encephalopathy may occur. Regular monitoring of clinical and laboratory parameters is required. + Phenytoin, and by extrapolation fosphenytoin Increased hyperammonemia with increased risk of encephalopathy may occur. Regular monitoring of clinical and laboratory parameters is required. + Propofol A possible increase in propofol blood levels may occur. When coadministered with valproate, a reduction in propofol dose should be considered. + Rifampicin There is a risk of seizures due to increased hepatic metabolism of valproate by rifampicin. Clinical monitoring and monitoring of laboratory parameters and possible anticonvulsant dose adjustment are required during treatment with rifampicin and after its discontinuation. + Rufinamide A possible increase in rufinamide concentrations may occur, in particular in children weighing less than 30 kg. In children weighing less than 30 kg: the total dose of 600 mg/day after dose titration should not be exceeded. + Topiramate Increased hyperammonemia with increased risk of encephalopathy may occur. Regular monitoring of clinical and laboratory parameters is required. + Zidovudine There is a risk of increased adverse effects of zidovudine, particularly hematological effects, due to decrease in its metabolism by valproic acid. Regular monitoring of clinical and laboratory parameters is required. A blood count should be performed to test for anemia during the first 2 months of the combination. + Zonisamide Increased hyperammonemia with increased risk of encephalopathy may occur. Regular monitoring of clinical and laboratory parameters is required. Other forms of interaction + Lithium Depalept has no effect on serum lithium levels. + Risk of liver damage The concomitant use of salicylates should be avoided in children under 3 years due to the risk of liver toxicity (see section 4.4). Concomitant use of valproate and other anticonvulsants increases the risk of liver damage, especially in young children (see section 4.4). Concomitant use with cannabidiol increases the incidence of raised transaminases. In patients of all ages receiving concomitantly cannabidiol at doses of 10 to 25 mg/kg and valproate, clinical trials have reported ALT increases greater than 3 times the upper limit of normal in 19% of patients. Appropriate liver monitoring should be exercised when valproate is used concomitantly with other anticonvulsants with potential hepatotoxicity, including cannabidiol. Dose reductions or therapy cessation should be considered in case of significant anomalies of liver parameters (see section 4.4).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
עלון מידע לצרכן
11.08.21 - עלון לצרכן 04.09.23 - עלון לצרכן אנגלית 29.08.21 - עלון לצרכן עברית 04.09.23 - עלון לצרכן עברית 04.09.23 - עלון לצרכן ערבית 03.01.23 - עלון לצרכן אנגלית 03.01.23 - עלון לצרכן ערבית 04.09.23 - עלון לצרכן אנגלית 04.09.23 - עלון לצרכן עברית 04.09.23 - עלון לצרכן ערבית 02.09.12 - החמרה לעלון 07.12.14 - החמרה לעלון 16.11.15 - החמרה לעלון 29.08.21 - החמרה לעלון 18.05.22 - החמרה לעלון 03.01.23 - החמרה לעלון 28.11.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
דפאלפט סירופ