Quest for the right Drug
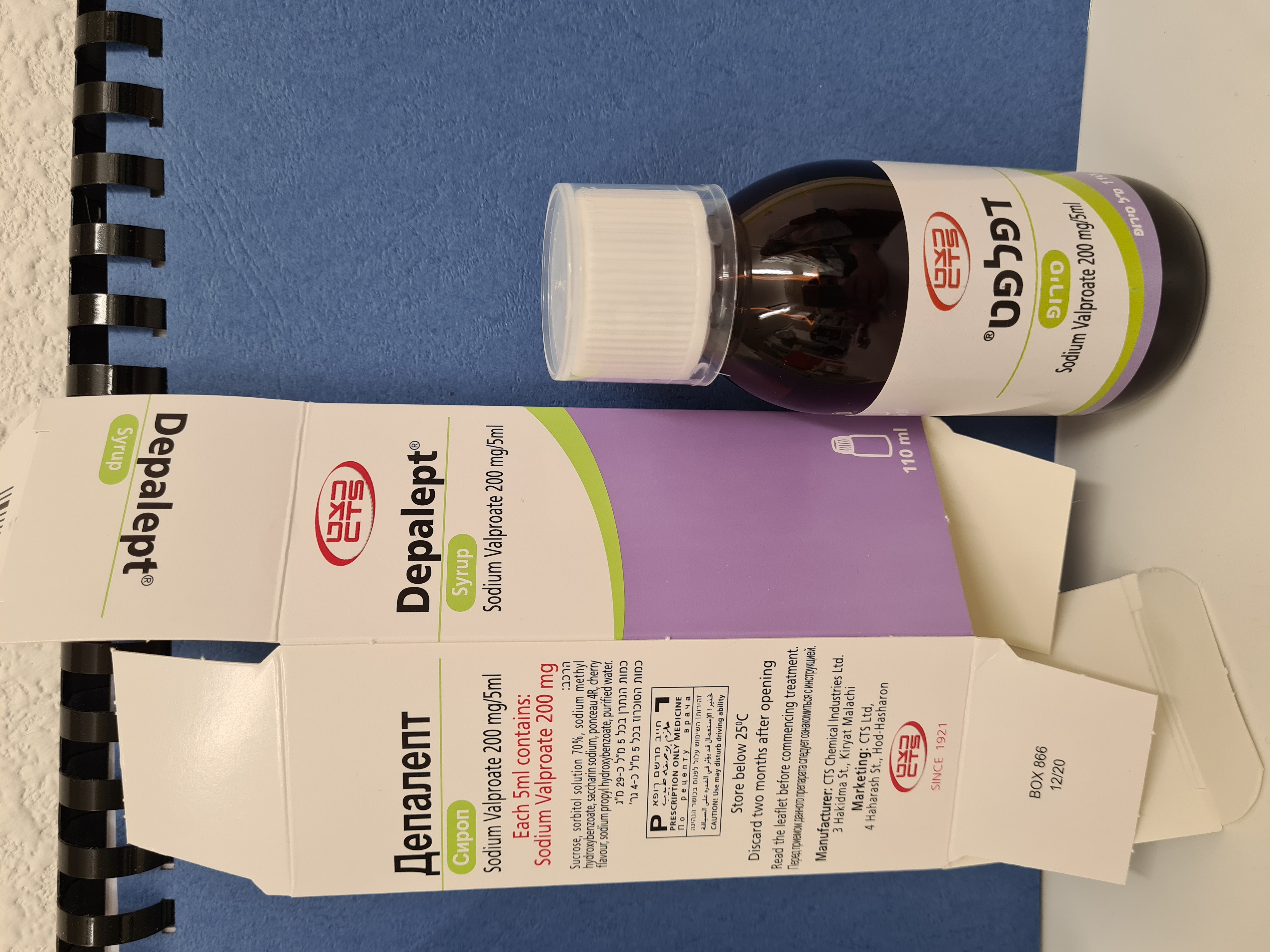
דפאלפט סירופ DEPALEPT SYRUP (VALPROIC ACID AS SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
סירופ : SYRUP
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4. Special warnings and precautions for use Special warnings Patient Card: This product is marketed with patient safety information card (patient card). Please explain to the patient the implications of this treatment. Female children/Female adolescents/Woman of childbearing potential/Pregnancy Valproate has a high teratogenic potential and children exposed in utero to valproate have a high risk for congenital malformations and neuro-developmental disorders (see section 4.6). Valproate should not be used in female children and women of childbearing potential unless other treatments are ineffective or not tolerated. If no other treatment is possible, the conditions below must be complied with. Depalept is contraindicated in the following situations: • In pregnancy unless there is no suitable alternative treatment (see sections 4.3 and 4.6). • In women of childbearing potential, unless the conditions listed below are fulfilled (see sections 4.3 and 4.6). Conditions The prescriber must ensure that: • individual circumstances should be evaluated in each case, involving the patient in the discussion, to guarantee her engagement, discuss therapeutic options and ensure her understanding of the risks and the measures needed to minimize the risks. • the potential for pregnancy is assessed for all female patients. • the patient has understood and acknowledged the risks of congenital malformations and neuro-developmental disorders including the magnitude of these risks for children exposed to valproate in utero. • the patient understands the need to undergo pregnancy testing prior to initiation of treatment and during treatment, as needed. • the patient is counseled regarding contraception, and that the patient is capable of complying with the need to use effective contraception (for further details please refer to subsection contraception of this boxed warning), without interruption during the entire duration of treatment with valproate. • the patient understands the need for regular (at least annual) review of treatment by a specialist experienced in the management of epilepsy. • the patient understands the need to consult her physician as soon as she is planning pregnancy to ensure timely discussion and switching to alternative treatment options prior to conception, and before contraception is discontinued. • the patient understands the need to urgently consult her physician in case of pregnancy. • the patient has received the Patient Guide. • the patient has acknowledged that she has understood the hazards and necessary precautions associated with valproate use. These conditions also concern women who are not currently sexually active unless the prescriber considers that there are compelling reasons to indicate that there is no risk of pregnancy. Female children • The prescriber must ensure that parents/caregivers of female children understand the need to contact the specialist once the female child using valproate experiences menarche. • The prescriber must ensure that parents/caregivers of female children who have experienced menarche are provided with comprehensive information about the risks of congenital malformations and neuro-developmental disorders including the magnitude of these risks for children exposed to valproate in utero. • In patients who experienced menarche, the prescribing specialist must reassess the need for valproate therapy annually and consider alternative treatment options. If valproate is the only suitable treatment, the need for using effective contraception and all other conditions listed should be discussed. Every effort should be made by the specialist to switch the female children to alternative treatment before they reach puberty or adulthood. Pregnancy test Pregnancy must be excluded before start of treatment with valproate. Treatment with valproate must not be initiated in women of childbearing potential without a negative result from a plasma pregnancy test with a sensitivity of at least 25 mIU/mL, confirmed by a healthcare provider, to rule out unintended use in pregnancy. This pregnancy test must be repeated at regular intervals during treatment. Contraception Women of childbearing potential who are prescribed valproate must use effective contraception, without interruption during the entire duration of treatment with valproate. These patients must be provided with comprehensive information on pregnancy prevention and should be referred for contraceptive advice if they are not using effective contraception. At least 1 effective method of contraception (preferably a user-independent form such as an intra-uterine device or implant) or 2 complementary forms of contraception including a barrier method should be used. Individual circumstances should be evaluated in each case when choosing the contraception method, involving the patient in the discussion, to guarantee her engagement and compliance with the chosen measures. Even if she has amenorrhea she must follow all the advice on effective contraception. Estrogen-containing products Concomitant use with estrogen-containing products, including estrogen-containing hormonal contraceptives, may result in decreased valproate efficacy (see section 4.5). Prescribers should monitor clinical response (seizure control) when initiating or discontinuing estrogen-containing products. However, valproate does not reduce efficacy of hormonal contraceptives. Annual treatment reviews by a specialist The specialist should at least annually review whether valproate is the most suitable treatment for the patient. The specialist should discuss the hazards and necessary precautions associated with valproate use at initiation and during each annual review and ensure that the patient has understood its content. Pregnancy planning If a woman is planning to become pregnant, a specialist experienced in the management of epilepsy must reassess valproate therapy and consider alternative treatment options. Every effort should be made to switch to appropriate alternative treatment prior to conception, and before contraception is discontinued (see section 4.6). If switching is not possible, the woman should receive further counseling regarding the valproate risks for the unborn child to support her informed decision-making regarding family planning. In case of pregnancy If a woman using valproate becomes pregnant, she must be immediately referred to a specialist to re-evaluate treatment with valproate and consider alternative options. The patients with a valproate-exposed pregnancy and their partners should be referred to a specialist experienced in teratology for evaluation and counseling regarding the exposed pregnancy (see section 4.6). Pharmacists must ensure that • the Patient Card is provided with every valproate dispensing and that the patients understand its content. • the patients are advised not to stop valproate medication and to immediately contact a specialist in case of planned or suspected pregnancy. Educational materials In order to assist healthcare professionals and patients in avoiding exposure to valproate during pregnancy, the Marketing Authorization Holder has provided educational materials to reinforce the warnings concerning the teratogenicity (congenital malformations) and fetotoxicity (neuro- developmental disorders) of valproate and provide guidance regarding use of valproate in women of childbearing potential. A Patient Card should be provided to all women of childbearing potential using valproate. Exacerbation of seizures As with other antiepileptics, administration of valproate may, instead of improvement, lead to a reversible exacerbation of seizure frequency and severity (including status epilepticus), or the onset of a new type of seizure. Patients should be advised to consult their physician immediately if exacerbation of seizures occurs (see section 4.8). These seizures should be differentiated from those that may occur due to a pharmacokinetic interaction (see section 4.5), toxicity (liver disease or encephalopathy - see sections 4.4 and 4.8) or overdose. Since this medicinal product is metabolized into valproic acid, it should not be combined with other medicinal products undergoing the same transformation to avoid an overdose of valproic acid (e.g. valproate semisodium, valpromide). Severe Liver damage Conditions of occurrence Severe liver damage resulting sometimes in fatalities has exceptionally been reported. Patients most at risk are infants and young children under the age of 3 years with severe seizure disorders, particularly those with brain damage, mental retardation and/or congenital metabolic or degenerative disease. After the age of 3 years, the risk is significantly reduced and it progressively decreases with age. In most cases, such liver damage occurred during the first 6 months of therapy, usually between the 2nd and 12th week and generally during antiepileptic polytherapy. Suggestive signs Clinical symptoms are essential for early diagnosis. In particular, the 2 conditions which may precede jaundice should be taken into consideration, especially in patients at risk (see “Conditions of occurrence”): • firstly, non-specific symptoms, usually of sudden onset, such as asthenia, anorexia, lethargy, drowsiness, which are sometimes associated with repeated vomiting and abdominal pain. • secondly, recurrence of epileptic seizures despite proper treatment compliance. Patients (or their family for children) should be instructed to report immediately any such signs to a physician should they occur. Investigations including clinical examination and laboratory assessment of liver functions should be undertaken immediately. Detection Liver function tests should be performed before therapy and regularly during the first 6 months of therapy, especially in patients at risk. If concomitant treatments known for their liver toxicity are changed (dose increase or new treatment), liver function tests must be carried out again 1(see also section 4.5 on the risk of liver damage with salicylates, other anticonvulsants including cannabidiol). Among the usual investigations, tests which reflect protein synthesis particularly prothrombin rate, are most relevant. Confirmation of an abnormally low prothrombin rate, particularly in association with other biological abnormalities (significant decrease in fibrinogen and coagulation factors; increased bilirubin level and raised transaminases – "Precautions for use") requires cessation of therapy with this medicinal product (as a matter of precaution and in case they are taken concomitantly, salicylate derivatives should also be discontinued since they follow the same metabolic pathway). Pancreatitis Pancreatitis, which may result in fatalities, has been very rarely reported. Young children are at particular risk but this can be observed irrespective of age and treatment duration. Pancreatitis with an unfavorable outcome is generally observed in young children or in patients with severe seizures, neurological impairment or anticonvulsant polytherapy. Hepatic failure with pancreatitis increases the risk of fatal outcome. In the event of acute abdominal pain or gastrointestinal signs such as nausea, vomiting and/or anorexia, a diagnosis of pancreatitis must be considered and, in patients with elevated pancreatic enzymes, treatment discontinued, and the necessary alternative therapeutic measures implemented. Suicidal ideation and behaviour Suicidal ideation and behavior have been reported in patients treated with antiepileptics in several indications. A meta-analysis of data from randomized, placebo-controlled trials of antiepileptic drugs has also shown a slight increase in risk of suicidal ideation and behavior. The causes of this risk are unknown and the available data do not make it possible to rule out an increased risk with valproate. Therefore, patients should be closely monitored for signs of suicidal ideation and behavior, and appropriate treatment should be considered. Patients (and caregivers of patients) should be advised to seek medical advice should signs of suicidal ideation or behavior emerge. Patients with known or suspected mitochondrial disease Valproate may trigger or worsen clinical signs of underlying mitochondrial diseases caused by mutations of mitochondrial DNA as well as the nuclear gene encoding the mitochondrial enzyme polymerase gamma (POLG). In particular, acute liver failure and liver-related deaths have been associated with valproate treatment at a higher rate in patients with hereditary neurometabolic syndromes caused by mutations in the POLG gene, e.g. Alpers-Huttenlocher Syndrome. POLG-related disorders should be suspected in patients with a family history or suggestive symptoms of a POLG-related disorder, including but not limited to unexplained encephalopathy, refractory epilepsy (focal, myoclonic), status epilepticus at presentation, developmental delays, psychomotor regression, axonal sensorimotor neuropathy, myopathy, cerebellar ataxia, ophthalmoplegia, or complicated migraine with occipital aura. POLG mutation testing should be performed in accordance with current clinical practice for the diagnostic evaluation of such disorders (see section 4.3). Interaction with other medicinal products Concomitant use of this medicinal product with lamotrigine and/or penems (carbapenems) is not recommended (see section 4.5). Cognitive or extrapyramidal disorders Cognitive or extrapyramidal disorders can be associated with imaging findings of cerebral atrophy. This type of clinical picture can thus be confused with dementia or Parkinson’s disease. These disorders are reversible on treatment discontinuation (see section 4.8). Information related to excipients Depalept 200, Depalept 500, Depalept Syrup and Depalept oral Solution contains 28 mg, 70mg, 29 mg and 28 sodium respectively per tablet, 5ml and 1 ml respectively equivalent to 1.4%, 3.5%, 1.5% and 1.4% respectively of the WHO recommended maximum daily intake of 2 g sodium for an adult. Depalept Syrup containes sorbitol and sucrose. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine. Precautions for use Liver function tests should be performed before starting therapy (see section 4.3) and then periodically during the first 6 months, particularly in patients at risk (see section 4.4 "Severe liver damage - Detection"). It should be emphasized that, as with most antiepileptic drugs, a moderate, transient and isolated increase in liver enzymes may be noted without any clinical signs, particularly at the beginning of the therapy. More extensive biological investigations (including prothrombin rate) are recommended in this case; an adjustment of dosage may be considered when appropriate and tests should be repeated as necessary. Blood tests (blood cell count, including platelet count, bleeding time and coagulation parameters) are recommended prior to treatment, then after 15 days and at the end of treatment or before surgery, and in case of spontaneous bruising or bleeding (see section 4.8). In patients with renal insufficiency, elevated circulating valproic acid concentrations in the blood should be taken into account and the dosage should be reduced accordingly. This medicinal product is contraindicated in patients with urea cycle enzyme deficiencies. A few cases of hyperammonemia with stupor or coma have been described in these patients (see section 4.3). Although immune disorders have been noted only exceptionally during the use of this medicinal product, its potential benefit should be weighed against its potential risk in patients with systemic lupus erythematosus. Patients should be warned of the risk of weight gain at the initiation of therapy and appropriate strategies should be adopted to minimize the risk. Since valproate is excreted mainly in the urine partly in the forms of ketone bodies, ketone body excretion test may give false positive results in diabetic patients. Patients with carnitine palmitoyltransferase (CPT) type II deficiency should be warned of the greater risk of rhabdomyolysis when taking valproate. Alcohol intake is not recommended during treatment with Depalept. Children Monotherapy is recommended in children under the age of 3 years when prescribing valproate, but the potential benefit should be weighed against the risk of liver damage or pancreatitis in such patients prior to initiation of therapy (see section 4.4 "Severe liver damage" and also section 4.5). The simultaneous prescription of salicylates should be avoided in children under 3 years due to the risk of hepatotoxicity (see also section 4.4) and the risk of bleeding. In children with a history of unexplained hepatic and gastrointestinal disorders (anorexia, vomiting, acute episodes of cytolysis), episodes of lethargy or coma, mental retardation or with a family history of neonatal or infant death, metabolic tests and, in particular, fasting and post-prandial blood ammonia tests must be performed prior to any valproate treatment.
Effects on Driving

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
עלון מידע לצרכן
11.08.21 - עלון לצרכן 04.09.23 - עלון לצרכן אנגלית 29.08.21 - עלון לצרכן עברית 04.09.23 - עלון לצרכן עברית 04.09.23 - עלון לצרכן ערבית 03.01.23 - עלון לצרכן אנגלית 03.01.23 - עלון לצרכן ערבית 04.09.23 - עלון לצרכן אנגלית 04.09.23 - עלון לצרכן עברית 04.09.23 - עלון לצרכן ערבית 02.09.12 - החמרה לעלון 07.12.14 - החמרה לעלון 16.11.15 - החמרה לעלון 29.08.21 - החמרה לעלון 18.05.22 - החמרה לעלון 03.01.23 - החמרה לעלון 28.11.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
דפאלפט סירופ