Quest for the right Drug
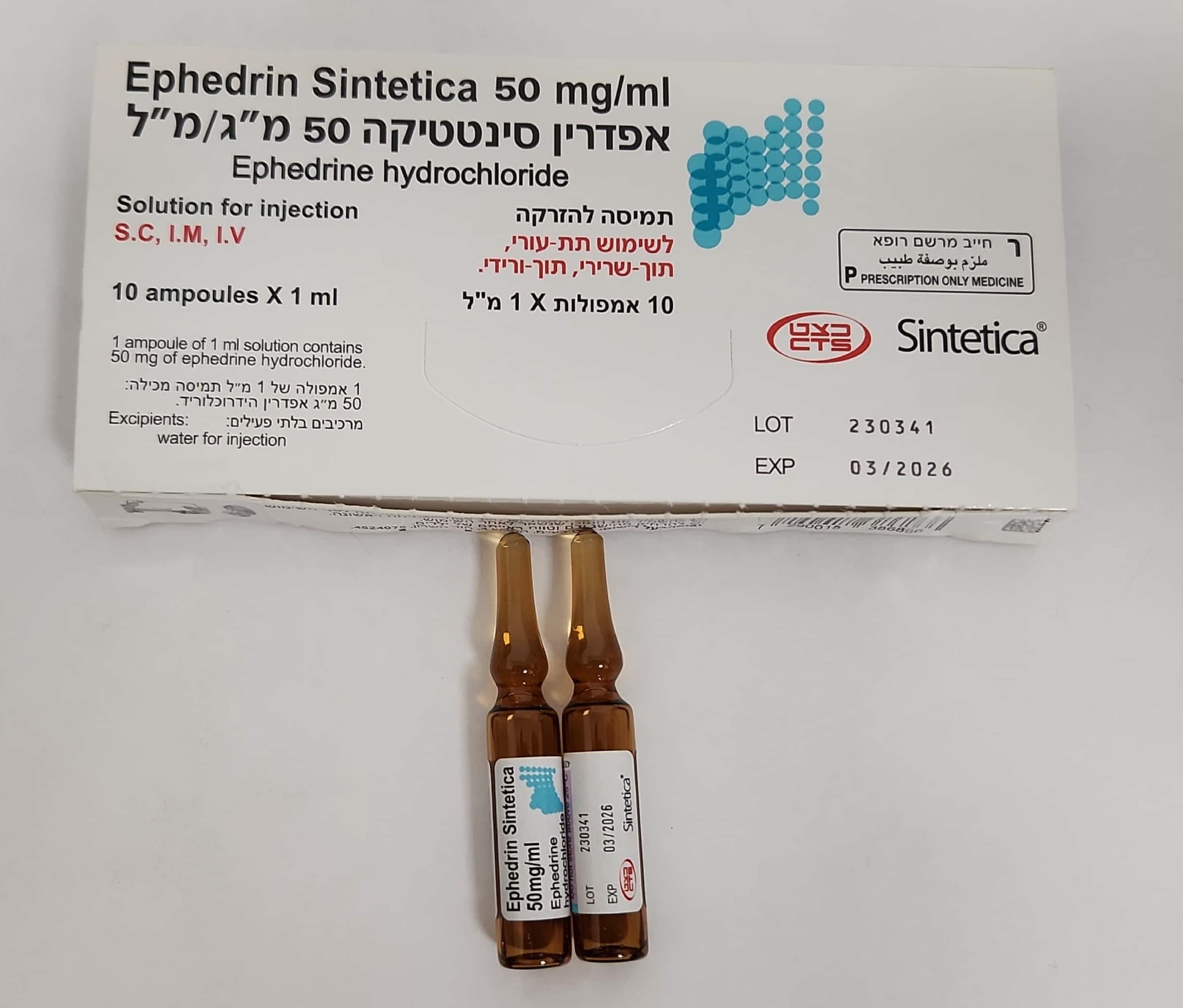
אפדרין סינטטיקה 50 מ"ג/מ"ל EPHEDRIN SINTETICA 50 MG/ML (EPHEDRINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי, תוך-ורידי, תוך-שרירי : S.C, I.V, I.M
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Adrenergic and Dopaminergic Agent. ATC Code: C01CA26 Ephedrine is a sympathomimetic amine acting directly on the alpha and beta receptors and indirectly by increasing the release of noradrenaline by the sympathetic nerve endings. As with any sympathomimetic agent, ephedrine stimulates the central nervous system, the cardiovascular system, the respiratory system, and the sphincters of the digestive and urinary systems. Ephedrine can provoke an increase of glycemia. After intravenous injection of a dose between 10 and 25 mg, the cardiac effects persist for 1 hour.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Absorption Ephedrine is rapidly and completed absorbed after oral, intramuscular or subcutaneous administration. Ephedrine hydrochloride circulates freely in the plasma. Distribution Although specific information is lacking, ephedrine is presumed to cross the placenta and to distribute into milk. After injection, it is rapidly distributed in the body and accumulates in the liver, kidneys, lungs, spleen and brain. This accumulation results in high distribution volumes ranging between 122 and 320 liters. Biotransformation A small fraction of ephedrine is slowly metabolized in the liver by oxidative deamination, demethylation, aromatic hydroxylation, and conjugation. The metabolites are identified as p-hydroxyephedrine, p-hydroxynorephedrine, norephedrine, and conjugates of these compounds. Elimination Excretion depends on urine pH: From 73 to 99% (mean: 88%) in acidic urine. From 22 to 35% (mean: 27%) in alkaline urine. After oral or parenteral administration, 77% of ephedrine is excreted in unchanged form in the urine. The half life depends on urine pH. In acidic urine at pH = 5, the half life is 3 hours; in alkaline urine at pH = 6.3, the half life is approximately 6 hours.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
