Quest for the right Drug
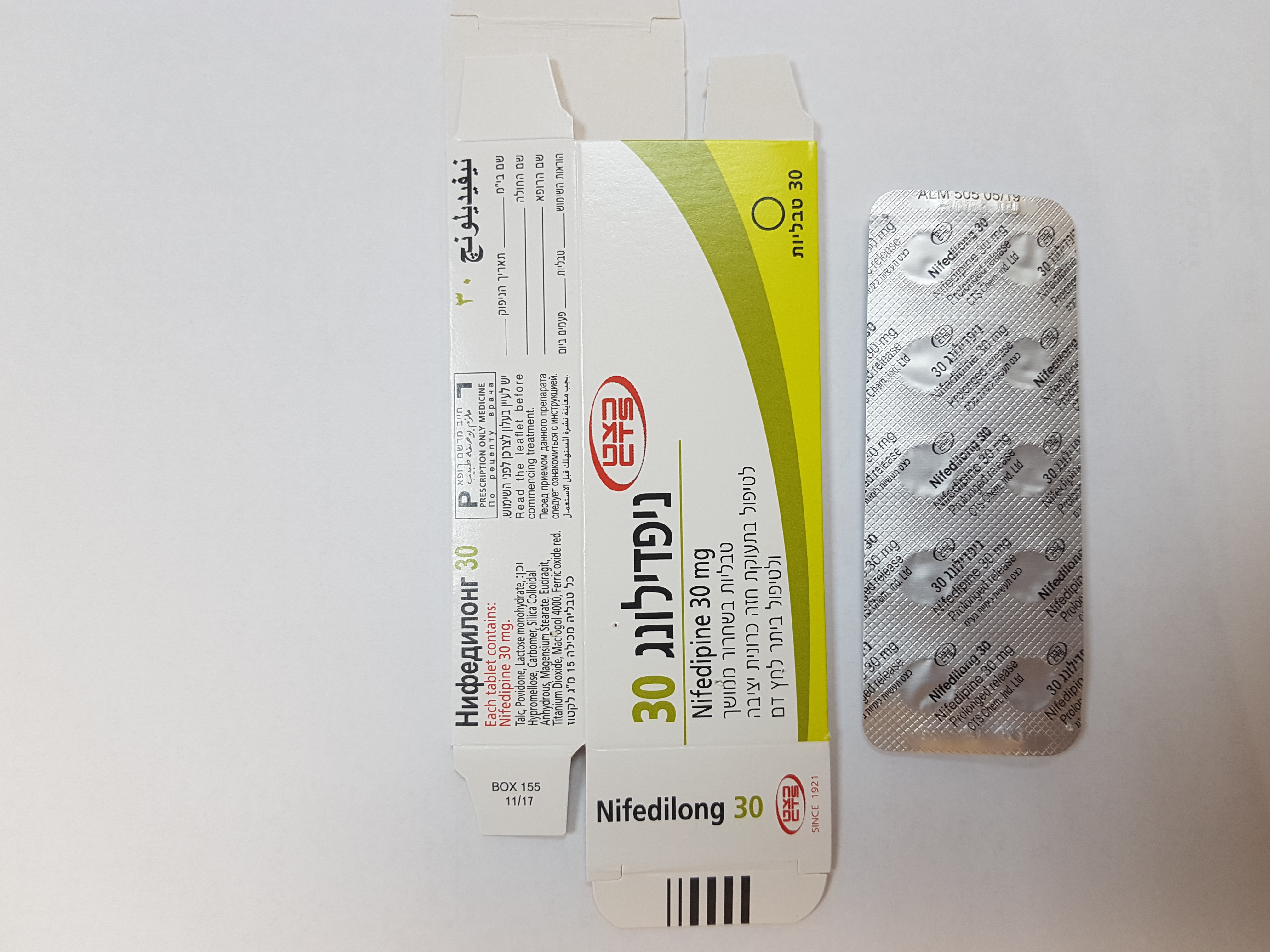
ניפדילונג 30 NIFEDILONG 30 (NIFEDIPINE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות בשחרור ממושך : TABLETS PROLONGED RELEASE
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Nifedilong tablets must be swallowed whole; under no circumstances should they be bitten, chewed or broken up. Caution should be exercised in patients with hypotension as there is a risk of further reduction in blood pressure and care must be exercised in patients with very low blood pressure (severe hypotension with systolic blood pressure less than 90 mm Hg). Nifedilong should not be used during pregnancy unless the clinical condition of the woman requires treatment with nifedipine. Nifedilong should be reserved for women with severe hypertension who are unresponsive to standard therapy (see section 4.6). Careful monitoring of blood pressure must be exercised when administering nifedipine with I.V. magnesium sulfate, owing to the possibility of an excessive fall in blood pressure, which could harm both mother and foetus. For further information regarding use in pregnancy, refer to section 4.6. Nifedilong is not recommended for use during breast-feeding because nifedipine has been reported to be excreted in human milk and the effects of nifedipine exposure to the infant are not known (see section 4.6). In patients with impaired liver function careful monitoring and, in severe cases, a dose reduction may be necessary. Nifedilong may be used in combination with beta-blocking drugs and other antihypertensive agents but the possibility of an additive effect resulting in postural hypotension should be borne in mind. Nifedilong will not prevent possible rebound effects after cessation of other antihypertensive therapy. Nifedilong should be used with caution in patients whose cardiac reserve is poor. Deterioration of heart failure has occasionally been observed with nifedipine. Diabetic patients taking Nifedilong may require adjustment of their control. In dialysis patients with malignant hypertension and hypovolaemia, a marked decrease in blood pressure can occur. Nifedipine is metabolised via the cytochrome P450 3A4 system. Drugs that are known to either inhibit or to induce this enzyme system may therefore alter the first pass or the clearance of nifedipine (see section 4.5). Drugs, which are known inhibitors of the cytochrome P450 3A4 system, and which may therefore lead to increased plasma concentrations of nifedipine include, for example: - macrolide antibiotics (e.g., erythromycin) - anti-HIV protease inhibitors (e.g., ritonavir) - azole antimycotics (e.g., ketoconazole) - the antidepressants, nefazodone and fluoxetine - quinupristin/dalfopristin - valproic acid - cimetidine Upon co-administration with these drugs, the blood pressure should be monitored and, if necessary, a reduction of the nifedipine dose should be considered. As the outer membrane of the Nifedilong tablet is not digested, what appears to be the complete tablet may be seen in the toilet or associated with the patient’s stools. Also, as a result of this, care should be exercised when administering Nifedilong to patients, as obstructive symptoms may occur. Bezoars can occur in very rare cases and may require surgical intervention. In single cases, obstructive symptoms have been described without known history of gastrointestinal disorders. A false positive effect may be experienced when performing a barium contrast x- ray. For use in special populations see section 4.2.
Effects on Driving
4.7 Effects on ability to drive and use machines Reactions to the drug, which vary in intensity from individual to individual, may impair the ability to drive or to operate machinery (see Section 4.8). This applies particularly at the start of treatment, on changing the medication and in combination with alcohol.

שימוש לפי פנקס קופ''ח כללית 1994
Hypertension, vasospastic angina (Prinzmetal), chronic stable angina
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
