Quest for the right Drug
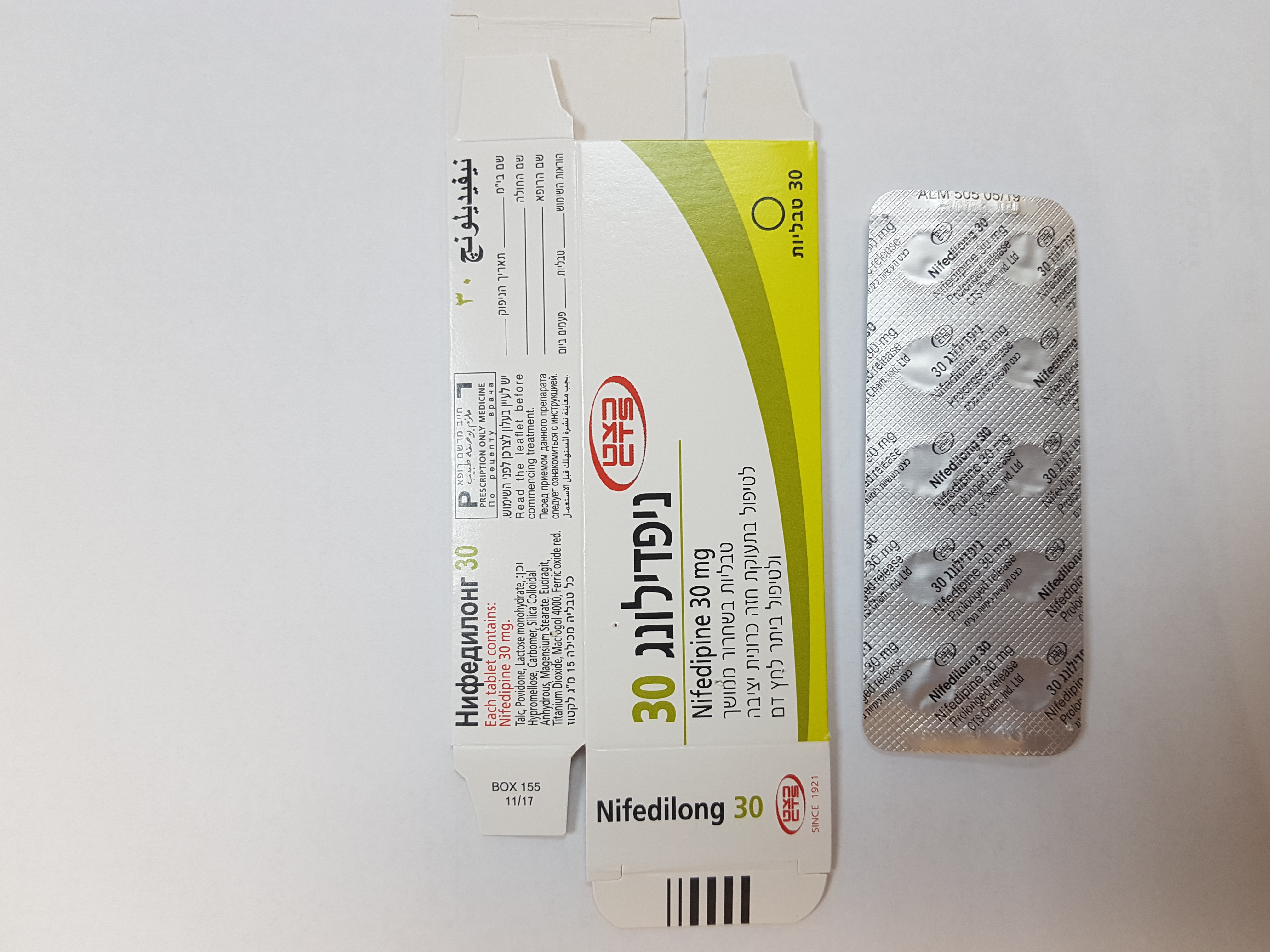
ניפדילונג 30 NIFEDILONG 30 (NIFEDIPINE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות בשחרור ממושך : TABLETS PROLONGED RELEASE
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pregnancy & Lactation : הריון/הנקה
4.6 Fertility, pregnancy and lactation Pregnancy Nifedipine should not be used during pregnancy unless the clinical condition of the woman requires treatment with nifedipine (see section 4.4). In animal studies, nifedipine has been shown to produce embryotoxicity, foetotoxicity and teratogenicity (see section 5.3). There are no adequate well controlled studies in pregnant women. From the clinical evidence available a specific prenatal risk has not been identified, although an increase in perinatal asphyxia, caesarean delivery, as well as prematurity and intrauterine growth retardation have been reported. It is unclear whether these reports are due to the underlying hypertension, its treatment, or to a specific drug effect. The available information is inadequate to rule out adverse drug effects on the unborn and newborn child. Therefore, any use in pregnancy requires a very careful individual risk benefit assessment and should only be considered if all other treatment options are either not indicated or have failed to be efficacious. Acute pulmonary oedema has been observed when calcium channel blockers, among others nifedipine, have been used as a tocolytic agent during pregnancy (see section 4.8), especially in cases of multiple pregnancy (twins or more), with the intravenous route and/or concomitant use of beta-2 agonists. Breast-feeding Nifedipine is excreted in the breast milk. The nifedipine concentration in the milk is almost comparable with mother serum concentration. For immediate release formulations, it is proposed to delay breast-feeding or milk expression for 3 to 4 hours after drug administration to decrease the nifedipine exposure to the infant (see section 4.4). Fertility In single cases of in vitro fertilisation calcium antagonists like nifedipine have been associated with reversible biochemical changes in the spermatozoa’s head section that may result in impaired sperm function. In those men who are repeatedly unsuccessful in fathering a child by in vitro fertilisation, and where no other explanation can be found, calcium antagonists like nifedipine should be considered as possible causes.

שימוש לפי פנקס קופ''ח כללית 1994
Hypertension, vasospastic angina (Prinzmetal), chronic stable angina
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
