Quest for the right Drug
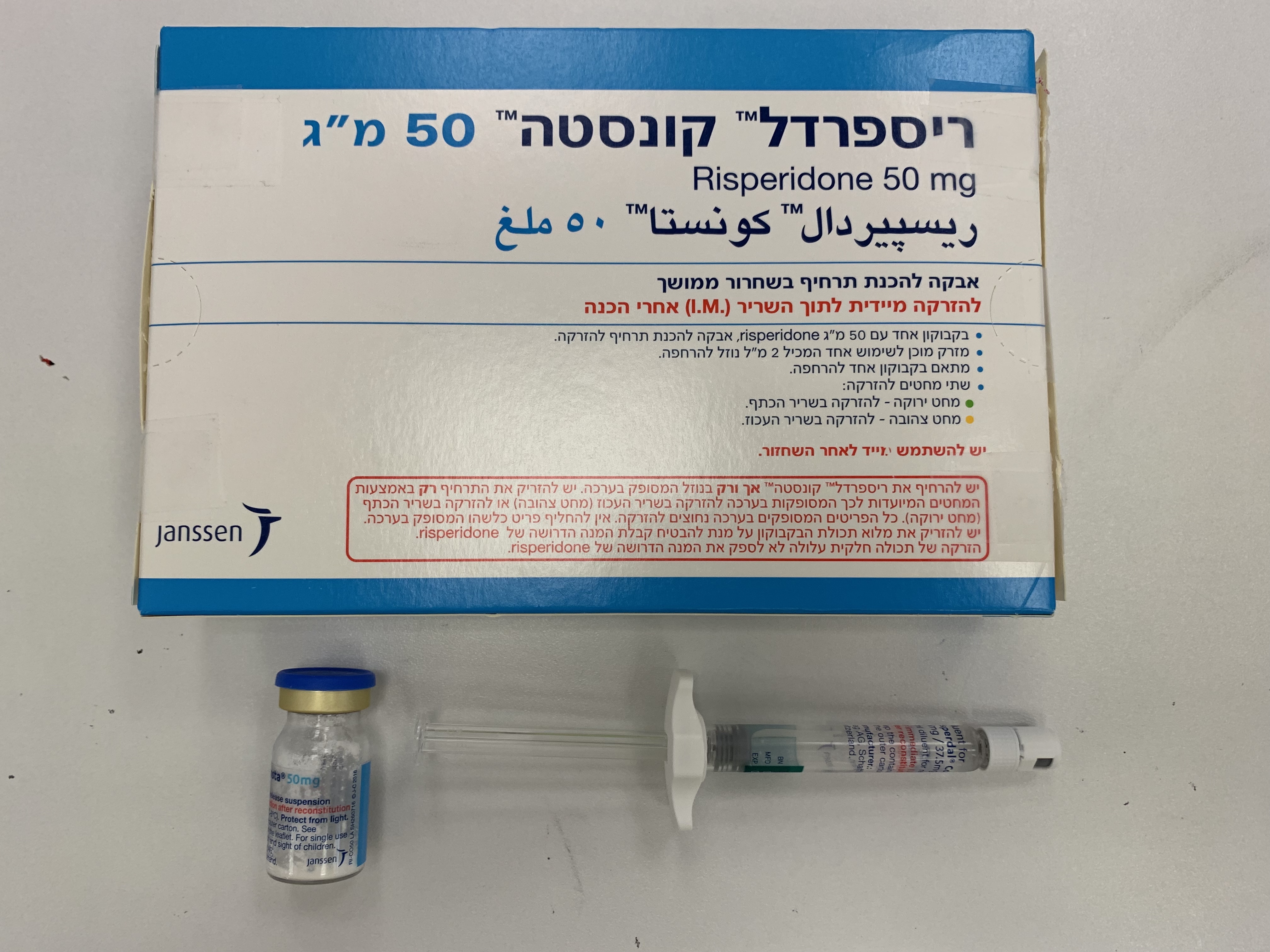
ריספרדל קונסטה 25 מ"ג RISPERDAL CONSTA 25 MG (RISPERIDONE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
אבקה לתרחיף להזרקה : POWDER FOR SUSPENSION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
2 DOSAGE AND ADMINISTRATION For patients who have never taken oral RISPERDAL®, it is recommended to establish tolerability with oral RISPERDAL® prior to initiating treatment with RISPERDAL CONSTA®. RISPERDAL CONSTA® should be administered every 2 weeks by deep intramuscular (IM) deltoid or gluteal injection. Each injection should be administered by a health care professional using the appropriate enclosed safety needle [see Dosage and Administration (2.8)]. For deltoid administration, use the 1-inch needle alternating injections between the two arms. For gluteal administration, use the 2-inch needle alternating injections between the two buttocks. Do not administer intravenously. 2.1 Schizophrenia The recommended dose for the treatment of schizophrenia is 25 mg IM every 2 weeks. Although dose response for effectiveness has not been established for RISPERDAL CONSTA®, some patients not responding to 25 mg may benefit from a higher dose of 37.5 mg or 50 mg. The maximum dose should not exceed 50 mg RISPERDAL CONSTA® every 2 weeks. No additional benefit was observed with dosages greater than 50 mg RISPERDAL CONSTA®; however, a higher incidence of adverse effects was observed. The efficacy of RISPERDAL CONSTA® in the treatment of schizophrenia has not been evaluated in controlled clinical trials for longer than 12 weeks. Although controlled studies have not been conducted to answer the question of how long patients with schizophrenia should be treated with RISPERDAL CONSTA®, oral risperidone has been shown to be effective in delaying time to relapse in longer term use. It is recommended that responding patients be continued on treatment with RISPERDAL CONSTA® at the lowest dose needed. The physician who elects to use RISPERDAL CONSTA® for extended periods should periodically re-evaluate the long-term risks and benefits of the drug for the individual patient. 2.2 Bipolar Disorder The recommended dose for monotherapy or adjunctive therapy to lithium or valproate for the maintenance treatment of Bipolar I Disorder is 25 mg IM every 2 weeks. Some patients may benefit from a higher dose of 37.5 mg or 50 mg. Dosages above 50 mg have not been studied in this population. The physician who elects to use RISPERDAL CONSTA® for extended periods should periodically re-evaluate the long-term risks and benefits of the drug for the individual patient. 2.3 General Dosing Information A lower initial dose of 12.5 mg may be appropriate when clinical factors warrant dose adjustment, such as in patients with hepatic or renal impairment, for certain drug interactions that increase risperidone plasma concentrations [see DRUG INTERACTIONS (7.11)] or in patients who have a history of poor tolerability to psychotropic medications. The efficacy of the 12.5 mg dose has not been investigated in clinical trials. Oral RISPERDAL® (or another antipsychotic medication) should be given with the first injection of RISPERDAL CONSTA® and continued for 3 weeks (and then discontinued) to ensure that adequate therapeutic plasma concentrations are maintained prior to the main release phase of risperidone from the injection site [see Clinical Pharmacology (12.3)]. Upward dose adjustment should not be made more frequently than every 4 weeks. The clinical effects of this dose adjustment should not be anticipated earlier than 3 weeks after the first injection with the higher dose. In patients with clinical factors such as hepatic or renal impairment or certain drug interactions that increase risperidone plasma concentrations [see DRUG INTERACTIONS (7.11)], dose reduction as low as 12.5 mg may be appropriate. The efficacy of the 12.5 mg dose has not been investigated in clinical trials. Do not combine two different dose strengths of RISPERDAL CONSTA® in a single administration. 2.4 Dosage in Special Populations Elderly For elderly patients treated with RISPERDAL CONSTA®, the recommended dosage is 25 mg IM every 2 weeks. Oral RISPERDAL® (or another antipsychotic medication) should be given with the first injection of RISPERDAL CONSTA® and should be continued for 3 weeks to ensure that adequate therapeutic plasma concentrations are maintained prior to the main release phase of risperidone from the injection site [see Clinical Pharmacology (12.3)]. Renal or Hepatic Impairment Patients with renal or hepatic impairment should be treated with titrated doses of oral RISPERDAL® prior to initiating treatment with RISPERDAL CONSTA®. The recommended starting dose is 0.5 mg oral RISPERDAL® twice daily during the first week, which can be increased to 1 mg twice daily or 2 mg once daily during the second week. If a total daily dose of at least 2 mg oral RISPERDAL® is well tolerated, an injection of 25 mg RISPERDAL CONSTA® can be administered every 2 weeks. Oral supplementation should be continued for 3 weeks after the first injection until the main release of risperidone from the injection site has begun. In some patients, slower titration may be medically appropriate. Alternatively, a starting dose of RISPERDAL CONSTA® of 12.5 mg may be appropriate. The efficacy of the 12.5 mg dose has not been investigated in clinical trials. Patients with renal impairment may have less ability to eliminate risperidone than normal adults. Patients with impaired hepatic function may have an increase in the free fraction of the risperidone, possibly resulting in an enhanced effect [see Clinical Pharmacology (12.3)]. Elderly patients and patients with a predisposition to hypotensive reactions or for whom such reactions would pose a particular risk should be instructed in nonpharmacologic interventions that help to reduce the occurrence of orthostatic hypotension (e.g., sitting on the edge of the bed for several minutes before attempting to stand in the morning and slowly rising from a seated position). These patients should avoid sodium depletion or dehydration, and circumstances that accentuate hypotension (alcohol intake, high ambient temperature, etc.). Monitoring of orthostatic vital signs should be considered [see Warnings and Precautions (5.7)]. 2.5 Reinitiation of Treatment in Patients Previously Discontinued There are no data to specifically address reinitiation of treatment. When restarting patients who have had an interval off treatment with RISPERDAL CONSTA®, supplementation with oral RISPERDAL® (or another antipsychotic medication) should be administered. 2.6 Switching from Other Antipsychotics There are no systematically collected data to specifically address switching patients from other antipsychotics to RISPERDAL CONSTA®, or concerning concomitant administration with other antipsychotics. Previous antipsychotics should be continued for 3 weeks after the first injection of RISPERDAL CONSTA® to ensure that therapeutic concentrations are maintained until the main release phase of risperidone from the injection site has begun [see Clinical Pharmacology (12.3)]. For patients who have never taken oral RISPERDAL®, it is recommended to establish tolerability with oral RISPERDAL® prior to initiating treatment with RISPERDAL CONSTA®. As recommended with other antipsychotic medications, the need for continuing existing EPS medication should be re evaluated periodically. 2.7 Co-Administration of RISPERDAL® CONSTA® with Certain Other Medications Co-administration of carbamazepine and other CYP 3A4 enzyme inducers (e.g., phenytoin, rifampin, phenobarbital) with risperidone would be expected to cause decreases in the plasma concentrations of the sum of risperidone and 9-hydroxyrisperidone combined, which could lead to decreased efficacy of RISPERDAL CONSTA® treatment. The dose of risperidone needs to be titrated accordingly for patients receiving these enzyme inducers, especially during initiation or discontinuation of therapy with these inducers [see Drug Interactions (7.11)]. At the initiation of therapy with carbamazepine or other known CYP 3A4 hepatic enzyme inducers, patients should be closely monitored during the first 4-8 weeks, since the dose of RISPERDAL CONSTA® may need to be adjusted. A dose increase, or additional oral RISPERDAL®, may need to be considered. On discontinuation of carbamazepine or other CYP 3A4 hepatic enzyme inducers, the dosage of RISPERDAL CONSTA® should be re-evaluated and, if necessary, decreased. Patients may be placed on a lower dose of RISPERDAL CONSTA® between 2 to 4 weeks before the planned discontinuation of carbamazepine or other CYP 3A4 inducers to adjust for the expected increase in plasma concentrations of risperidone plus 9- hydroxyrisperidone. For patients treated with the recommended dose of 25 mg RISPERDAL CONSTA® and discontinuing from carbamazepine or other CYP3A4 enzyme inducers, it is recommended to continue treatment with the 25-mg dose unless clinical judgment necessitates lowering the RISPERDAL CONSTA® dose to 12.5 mg or necessitates interruption of RISPERDAL CONSTA® treatment. The efficacy of the 12.5 mg dose has not been investigated in clinical trials. Fluoxetine and paroxetine, CYP 2D6 inhibitors, have been shown to increase the plasma concentration of risperidone 2.5-2.8 fold and 3-9 fold respectively. Fluoxetine did not affect the plasma concentration of 9- hydroxyrisperidone. Paroxetine lowered the concentration of 9- hydroxyrisperidone by about 10%. The dose of risperidone needs to be titrated accordingly when fluoxetine or paroxetine is co-administered. When either concomitant fluoxetine or paroxetine is initiated or discontinued, the physician should re-evaluate the dose of RISPERDAL CONSTA®. When initiation of fluoxetine or paroxetine is considered, patients may be placed on a lower dose of RISPERDAL CONSTA® between 2 to 4 weeks before the planned start of fluoxetine or paroxetine therapy to adjust for the expected increase in plasma concentrations of risperidone. When fluoxetine or paroxetine is initiated in patients receiving the recommended dose of 25 mg RISPERDAL CONSTA®, it is recommended to continue treatment with the 25 mg dose unless clinical judgment necessitates lowering the RISPERDAL CONSTA® dose to 12.5 mg or necessitates interruption of RISPERDAL CONSTA® treatment. When RISPERDAL CONSTA® is initiated in patients already receiving fluoxetine or paroxetine, a starting dose of 12.5 mg can be considered. The efficacy of the 12.5 mg dose has not been investigated in clinical trials. The effects of discontinuation of concomitant fluoxetine or paroxetine therapy on the pharmacokinetics of risperidone and 9-hydroxyrisperidone have not been studied. [see Drug Interactions (7.11)] 2.8 Instructions for Use For deltoid or gluteal intramuscular injection only Important Information RISPERDAL CONSTA® requires close attention to these step-by-step ‘Instructions for Use to help ensure successful administration. Use components provided The components in this dose pack are specifically designed for use with RISPERDAL CONSTA®. RISPERDAL CONSTA® must be reconstituted only in the diluent supplied in the dose pack. Do not substitute ANY components of the dose pack. Do not store suspension after reconstitution Administer dose as soon as possible after reconstitution to avoid settling. Proper dosing The entire contents of the vial must be administered to ensure intended dose of RISPERDAL CONSTA® is delivered. SINGLE-USE DEVICE Do not reuse. Medical devices require specific material characteristics to perform as intended. These characteristics have been verified for single use only. Any attempt to re-process the device for subsequent re-use may adversely affect the integrity of the device or lead to deterioration in performance. Dose pack contents Step 1 Assemble components Take out dose pack Connect vial adapter to vial Wait 30 minutes Remove cap from Prepare vial Connect vial adapter Remove dose pack vial adapter to vial from the refrigerator Flip off colored cap Hold sterile blister Place vial on a hard and allow to sit at from vial. as shown. Peel back surface and hold by the room temperature and remove paper base. Center vial adapter for at least 30 Wipe top of the grey backing. over the grey rubber minutes before stopper with an stopper. Push vial reconstituting. alcohol swab. Allow Do not remove vial adapter straight down to air dry. adapter from blister. onto vial top until it Do not warm any snaps securely into other way. Do not remove grey Do not touch spike place. rubber stopper. tip at any time. This will result in Do not place vial contamination. adapter on at an angle or diluent may leak upon transfer to the vial. Connect prefilled syringe to vial adapter Remove sterile Use proper grip Remove cap Connect syringe to blister Hold by white collar Holding the white vial adapter at the tip of the collar, snap off the Hold vial adapter by syringe. white cap. skirt to keep stationary. Do not hold syringe Do not twist or cut by the glass barrel off the white cap. Hold syringe by during assembly. white collar then Do not touch syringe insert tip into the luer tip. This will result in Keep vial vertical to opening of the vial contamination. prevent leakage. adapter. Hold base of vial and Do not hold the glass pull up on the sterile syringe barrel. This blister to remove. may cause the white collar to loosen or Do not shake. detach. The broken-off cap Do not touch can be discarded. Attach the syringe to exposed luer opening the vial adapter with on vial adapter. a firm clockwise This will result in twisting motion until contamination. it feels snug. Do not over-tighten. Over-tightening may cause the syringe tip to break. Step 2 Reconstitute microspheres Inject diluent Suspend Transfer suspension Remove vial Inject entire amount to syringe adapter microspheres in of diluent from diluent Invert vial Hold white collar on syringe into the vial. Continuing to hold completely. Slowly the syringe and down the plunger rod, pull plunger rod unscrew from vial shake vigorously for down to withdraw adapter. at least 10 seconds, as entire contents from the vial into the Tear section of the shown. vial label at the syringe. Check the suspension. perforation. Apply When properly mixed, detached label to the the suspension appears syringe for uniform, thick and identification milky in color. purposes. Microspheres will be Discard both vial visible in the liquid. and vial adapter Immediately proceed appropriately. to the next step so suspension does not settle. Step 3 Attach needle Select appropriate needle Attach needle Resuspend microspheres Choose needle based on Peel blister pouch open part Fully remove the blister injection location way and use to grasp the base pouch. (gluteal or deltoid). of the needle, as shown. Just before injection, shake Holding the white collar on syringe vigorously again, as the syringe, attach syringe to some settling will have needle luer connection with a occurred. firm clockwise twisting motion until snug. Do not touch needle luer opening. This will result in contamination. Step 4 Inject dose Remove Remove air Inject Secure needle in Properly dispose transparent bubbles Immediately inject safety device of needles needle protector Hold needle entire contents of Using one hand, Check to confirm Move the needle upright and tap syringe place needle safety needle safety safety device back gently to make intramuscularly device at a 45- device is fully towards the any air bubbles (IM) into the degree angle on a engaged. syringe, rise to the top. gluteal or deltoid hard, flat surface. Discard in an as shown. Then Slowly and muscle of the Press down with a approved sharps hold white collar carefully press patient. firm, quick motion container. on syringe and plunger rod until needle is fully Gluteal injection Also discard the carefully pull the upward to remove engaged in safety should be made unused needle transparent needle air. device. into the upper- provided in the protector straight outer quadrant of dose pack. off. the gluteal area. Avoid needle stick Do not twist injury: transparent needle Do not administer protector, as the intravenously. Do not use two luer connection hands. may loosen. Do not intentionally disengage or mishandle the needle safety device. Do not attempt to straighten the needle or engage the safety device if the needle is bent or damaged.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול בסכיזופרניה והפרעות סכיזואפקטיביות בהתקיים כל אלה: א. חולים שאושפזו בעבר ונקלעו לסיכון ממשי של אשפוז חוזר עקב אי הענות לנטילת התכשיר. ב. התחלת הטיפול בתרופה תהיה על פי הוראתו של מנהל מחלקה בבית חולים או מנהל מרפאה שהם רופאים מומחים בפסיכיאטריה או בפסיכיאטריה של הילד והמתבגר.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| סכיזופרניה והפרעות סכיזואפקטיביות |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
15/04/2005
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
