Quest for the right Drug
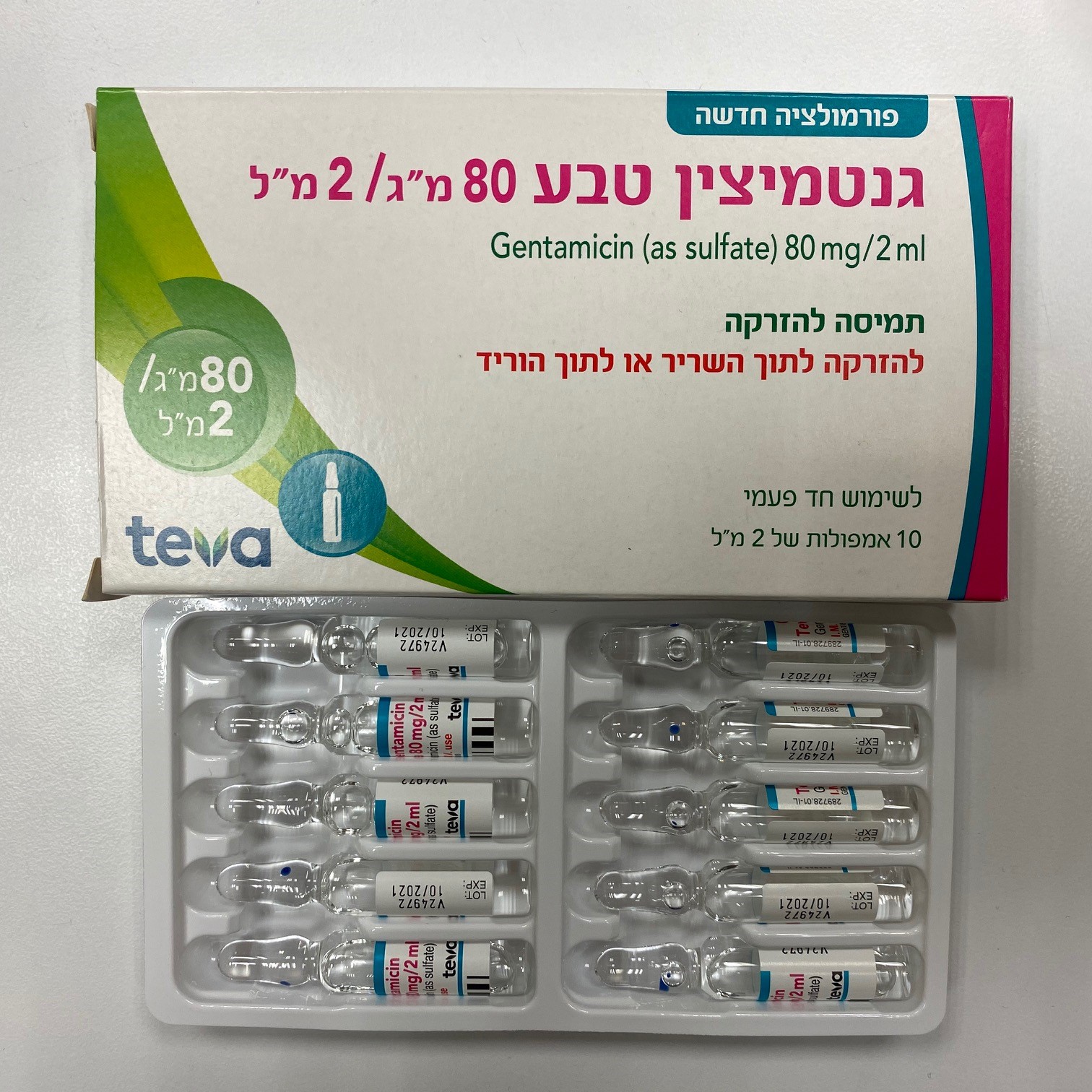
גנטמיצין טבע 80 מ"ג / 2 מ"ל GENTAMICIN TEVA 80 MG/2 ML (GENTAMICIN AS SULFATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי, תוך-שרירי : I.V, I.M
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. Pharmaceutical particulars 6.1 List of excipients Acetylcysteine, sodium hydroxide, edetate disodium, water for injection 6.2 Incompatibilities In general, gentamicin should not be mixed with other medicinal products. In particular the following are incompatible in mixed solution with gentamicin injection: beta-lactam antibiotics (e.g. penicillins, cephalosporins), erythromycin, or lipiphysan (a special oil-in- water-emulsion for parenteral nutrition) as this may cause physico-chemical inactivation. This also applies to a combination of gentamicin with diazepam, furosemide, flecainide acetate or heparin sodium. Dilution in the body will obviate the danger of physical and chemical incompatibility and enable gentamicin to be given concurrently with the drugs listed above either as a bolus injection into the drip tubing, with adequate flushing, or at separate sites. In the case of carbenicillin, administration should only be at a separate site. The following active substances or solution for reconstitution/dilution should not be administered simultaneously: Gentamicin is incompatible with amphotericin B, cephalothin sodium, nitrofurantoin sodium, sulfadiazine sodium and tetracyclines. Addition of gentamicin to solutions containing bicarbonate may lead to the release of carbon dioxide. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. Gentamicin Teva mixed with Sodium chloride 0.9% solution is stable for 24 hours at room temperature. However, from a microbiological point of view, the infusion preparation should be used immediately after preparation. If not used immediately, the in- use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2-8ºC. 6.4 Special precautions for storage Store below 25°C. For storage conditions after dilution of the medicinal product, see section 6.3. 6.5 Nature and contents of container Carton box containing 10 glass ampoules. Each ampoule contains 2 ml solution. 6.6 Special precautions for disposal and other handling For single use only. Before administration, a visual check must be made to ensure that the solution is free of foreign particles. Any unused medicinal product or waste material should be disposed of in accordance with local requirements. Manufacturer and License Holder Teva Israel Ltd., 124 Dvora HaNevi'a St., Tel Aviv 6944020.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
