Quest for the right Drug
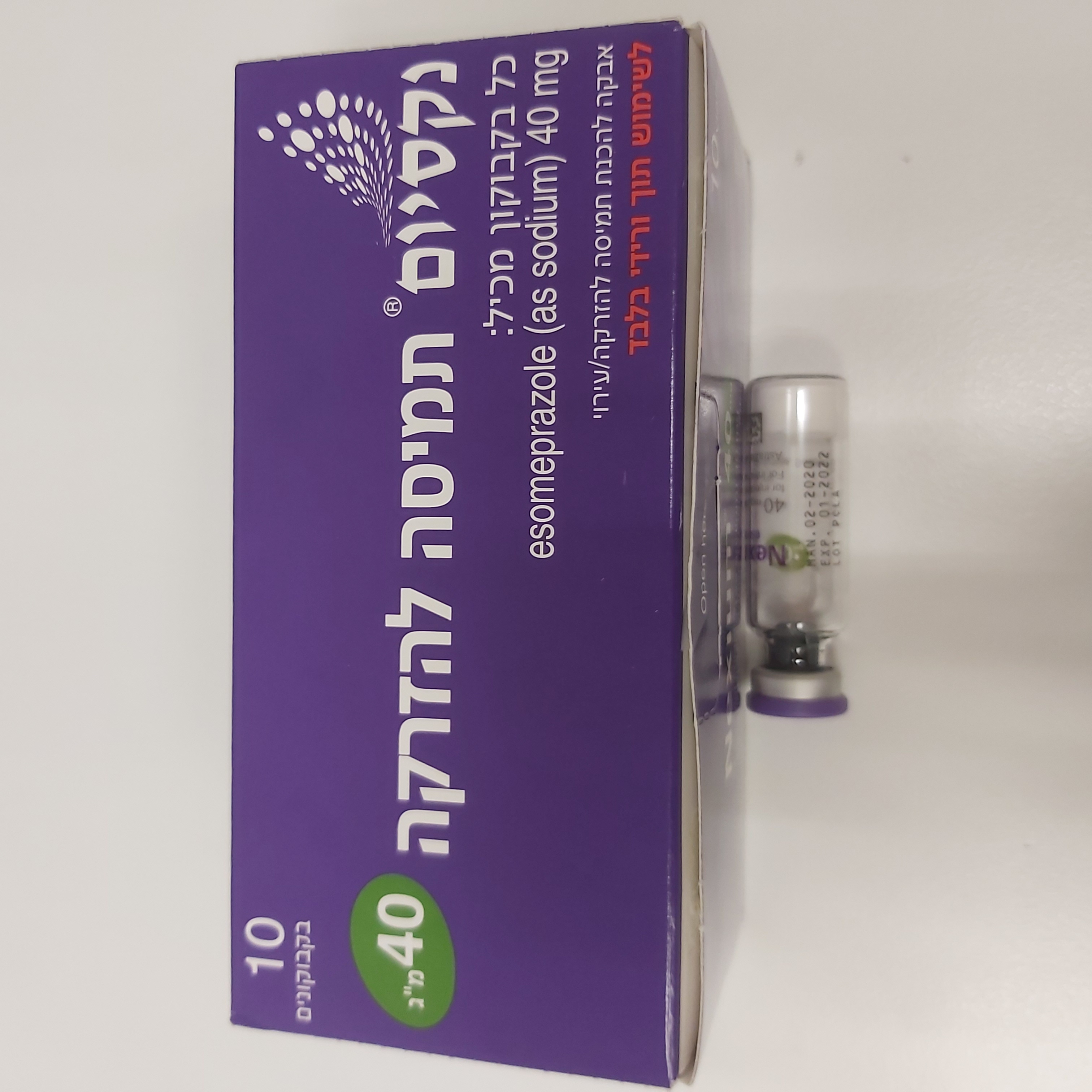
נקסיום אבקה לתמיסה להזרקה/ עירוי 40 מ"ג NEXIUM POWDER FOR SOLUTION FOR INJ/INF 40 MG (ESOMEPRAZOLE AS SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להמסה להזרקהאינפוזיה : POWDER FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile Headache, abdominal pain, diarrhoea and nausea are among those adverse reactions that have been most commonly reported in clinical trials (and also from post-marketing use). In addition, the safety profile is similar for different formulations, treatment indications, age groups and patient populations. No dose-related adverse reactions have been identified. Tabulated list of adverse reactions The following adverse medicinal product reactions have been identified or suspected in the clinical trials programme for esomeprazole administered orally or intravenously and post- marketing when administered orally. The reactions are classified according to frequency: very common >1/10; common >1/100 to <1/10; uncommon >1/1,000 to <1/100; rare>1/10,000 to <1/1,000; very rare <1/10,000; not known (cannot be estimated from the available data). System Organ Class Frequency Undesirable Effect Blood and lymphatic system Rare Leukopenia, thrombocytopenia disorders Very rare Agranulocytosis, pancytopenia Immune system disorders Rare Hypersensitivity reactions e.g. fever, angioedema and anaphylactic reaction/shock Metabolism and nutrition Uncommon Peripheral oedema disorders Rare Hyponatraemia Not known Hypomagnesaemia (see section 4.4); severe hypomagnesaemia can correlate with hypocalcaemia. Hypomagnesaemia may also be associated with hypokalaemia. Psychiatric disorders Uncommon Insomnia Rare Agitation, confusion, depression Very rare Aggression, hallucinations Nervous system disorders Common Headache Uncommon Dizziness, paraesthesia, somnolence Rare Taste disturbance Eye disorders Uncommon Blurred vision Ear and labyrinth disorders Uncommon Vertigo Respiratory, thoracic and Rare Bronchospasm mediastinal disorders Gastrointestinal disorders Common Abdominal pain, constipation, diarrhoea, flatulence, nausea/vomiting, fundic gland polyps (benign) Uncommon Dry mouth Rare Stomatitis, gastrointestinal candidiasis Not known Microscopic colitis Hepatobiliary disorders Uncommon Increased liver enzymes Rare Hepatitis with or without jaundice Very rare Hepatic failure, encephalopathy in patients with pre-existing liver disease Skin and subcutaneous Common Administration site reactions* tissue disorders Uncommon Dermatitis, pruritus, rash, urticaria Rare Alopecia, photosensitivity Very rare Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (TEN) Not known Subacute cutaneous lupus erythematosus (see section 4.4) Musculoskeletal and Uncommon Fracture of the hip, wrist or spine (see connective tissue disorders section 4.4) Rare Arthralgia, myalgia Very rare Muscular weakness Renal and urinary disorders Very rare Interstitial nephritis: in some patients, renal failure has been reported concomitantly Reproductive system and Very rare Gynaecomastia breast disorders General disorders and Rare Malaise, increased sweating administration site conditions *Administration site reactions have mainly been observed in a study with high-dose exposure over 3 days (72 hours) (see section 5.3). Irreversible visual impairment has been reported in isolated cases of critically ill patients who have received omeprazole (the racemate) intravenous injection, especially at high doses, but no causal relationship has been established. Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il Paediatric population A randomised, open-label, multi-national study was conducted to evaluate the pharmacokinetics of repeated intravenous doses for 4 days of once daily esomeprazole in paediatric patients 0 to 18 years old (see section 5.2). A total of 57 patients (8 children in the age group 1– 5 years) were included for safety evaluation. The safety results are consistent with the known safety profile of esomeprazole, and no new safety signals were identified.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לרופא
19.07.21 - עלון לרופאלתרופה במאגר משרד הבריאות
נקסיום אבקה לתמיסה להזרקה/ עירוי 40 מ"ג