Quest for the right Drug
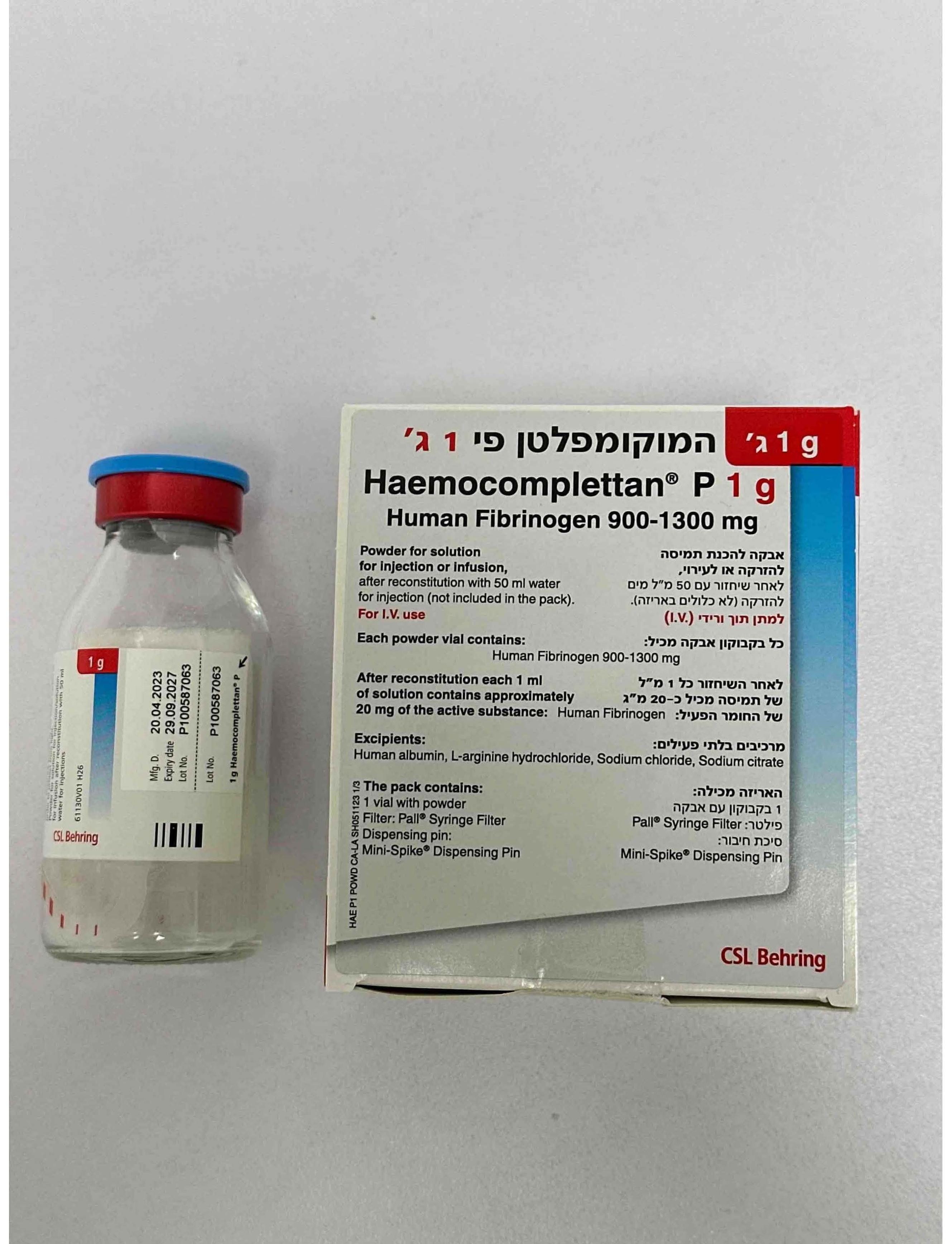
המוקומפלטן פי 1 ג' HAEMOCOMPLETTAN P 1 G (HUMAN FIBRINOGEN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להמסה להזרקהאינפוזיה : POWDER FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Tabulated list of adverse drug reactions (ADRs) This table combines the adverse reactions identified from clinical trials and post marketing experience. Frequencies presented in the table below have been based on pooled analyses across two company-sponsored clinical trials performed in aortic surgery with or without other surgical procedures [BI3023_2002 (N=61) and BI3023_3002 (N=152)] and have been evaluated according to the following convention: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000). For spontaneous post marketing ADRs, the reporting frequency is categorized as unknown. In view of the fact that these trials were conducted in only the narrow population of aortic surgery adverse drug reaction rates observed in these trials may not reflect the rates observed in clinical practice and are unknown for clinical settings outside the studied indication. MedDRA Undesirable effects Frequency System Organ Class (In aortic surgery with or without other surgical procedures) General Disorders and Pyrexia Very common Administration Site Condition Immune System Anaphylactic reactions Uncommon Disorder (including anaphylactic shock) Allergic reactions Unknown (including generalized urticaria, rash, dyspnoea, angioedema, tachycardia, nausea, vomiting, chills, pyrexia, chest pain, cough, blood pressure decreased) Vascular Disorder Thromboembolic events* Common** * Isolated cases have been fatal. ** Based on results of two clinical trials (aortic surgery with or without other surgical procedures), the pooled incidence rate of thromboembolic events was lower in fibrinogen treated subjects (N=8, 7.4 %) compared with placebo group (N=11, 10.4 %). For safety with respect to transmissible agents, see section 4.4. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/ and emailed to the Registration Holder’s Patient Safety Unit at: PV-IL@cslbehring.com

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
