Quest for the right Drug
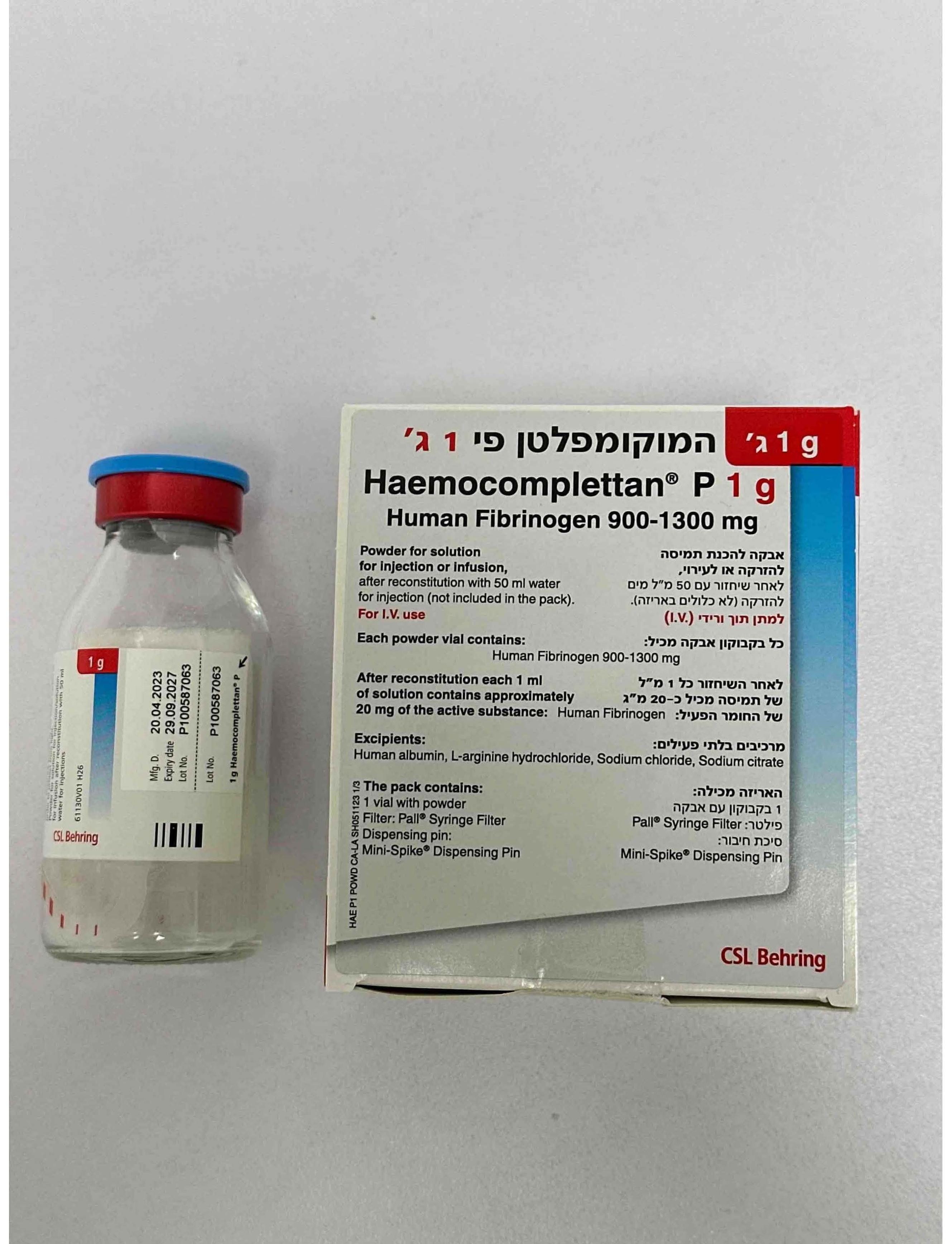
המוקומפלטן פי 1 ג' HAEMOCOMPLETTAN P 1 G (HUMAN FIBRINOGEN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להמסה להזרקהאינפוזיה : POWDER FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: antihaemorrhagics, human fibrinogen, ATC code: B02B B01 Human fibrinogen (coagulation factor I), in the presence of thrombin, activated coagulation factor XIII (F XIIIa) and calcium ions, is converted into a stable and elastic three- dimensional haemostatic fibrin clot. The administration of human fibrinogen concentrate causes an increase in plasma fibrinogen level and can temporarily correct the coagulation defect of patients with fibrinogen deficiency. The pivotal Phase II study evaluated the single-dose pharmacokinetics (see 5.2 Pharmacokinetic properties) and also provided efficacy data using the surrogate endpoint maximum clot firmness (MCF) and safety data. For each subject, the MCF was determined before (baseline) and one hour after a single dose administration of 70 mg/kg body weight of Haemocomplettan. Haemocomplettan was found to be effective in increasing clot firmness in patients with congenital fibrinogen deficiency (afibrinogenaemia) as measured by thromboelastometry. Haemostatic efficacy in acute bleeding episodes, and its correlation with MCF, are being verified in a postmarketing study.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Human fibrinogen is a normal constituent of the human plasma and acts like endogenous fibrinogen. In plasma, the biological half-life of fibrinogen is 3 to 4 days. Regarding degradation Haemocomplettan behaves like the endogenous fibrinogen. Haemocomplettan is administered intravenously and is immediately available in a plasma concentration corresponding to the dosage administered. A pharmacokinetic study evaluated the single-dose pharmacokinetics before and after administration of human fibrinogen concentrate in subjects with congenital afibrinogenaemia. This prospective, open label, uncontrolled, multicentre study consisted of 5 females and 10 males, ranging in age from 8 to 61 years (2 children, 3 adolescents, 10 adults). The median dose was 77.0 mg/kg body weight (range 76.6 – 77.4 mg/kg). Blood was sampled from 15 subjects (14 measurable) to determine the fibrinogen activity at baseline and up to 14 days after the infusion was complete. In addition, the incremental in vivo recovery (IVR, defined as the maximum increase in fibrinogen plasma levels per mg/kg body weight dosed), was determined from levels obtained up to 4 hours post-infusion. The median incremental IVR was 1.7 (range 1.30-2.73) mg/dl per mg/kg body weight. The following table provides the pharmacokinetic results: Pharmacokinetic results for fibrinogen activity Parameter (n=14) Mean ± SD Median (range) t1/2 [h] 78.7 ± 18.13 77.1 (55.73-117.26) Cmax [g/l] 1.4 ± 0.27 1.3 (1.00-2.10) AUC for dose of 70 mg/kg 124.3 ± 24.16 126.8 (81.73-156.40) [h•mg/ml] Extrapolated part of AUC [%] 8.4 ± 1.72 7.8 (6.13-12.14) Cl [ml/h/kg] 0.59 ± 0.13 0.55 (0.45-0.86) MRT [h] 92.8 ± 20.11 85.9 (66.14-126.44) Vss [ml/kg] 52.7 ± 7.48 52.7 (36.22-67.67) IVR [mg/dl per mg/kg bw] 1.8 ± 0.35 1.7 (1.30-2.73) t1/2 = terminal elimination half-life h = hour Cmax = maximum fibrinogen concentration in plasma within 4 hours AUC = area under the plasma concentration curve Cl = clearance MRT = mean residence time Vss = volume of distribution at steady state SD = standard deviation IVR = in vivo recovery bw= body weight

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
