Quest for the right Drug
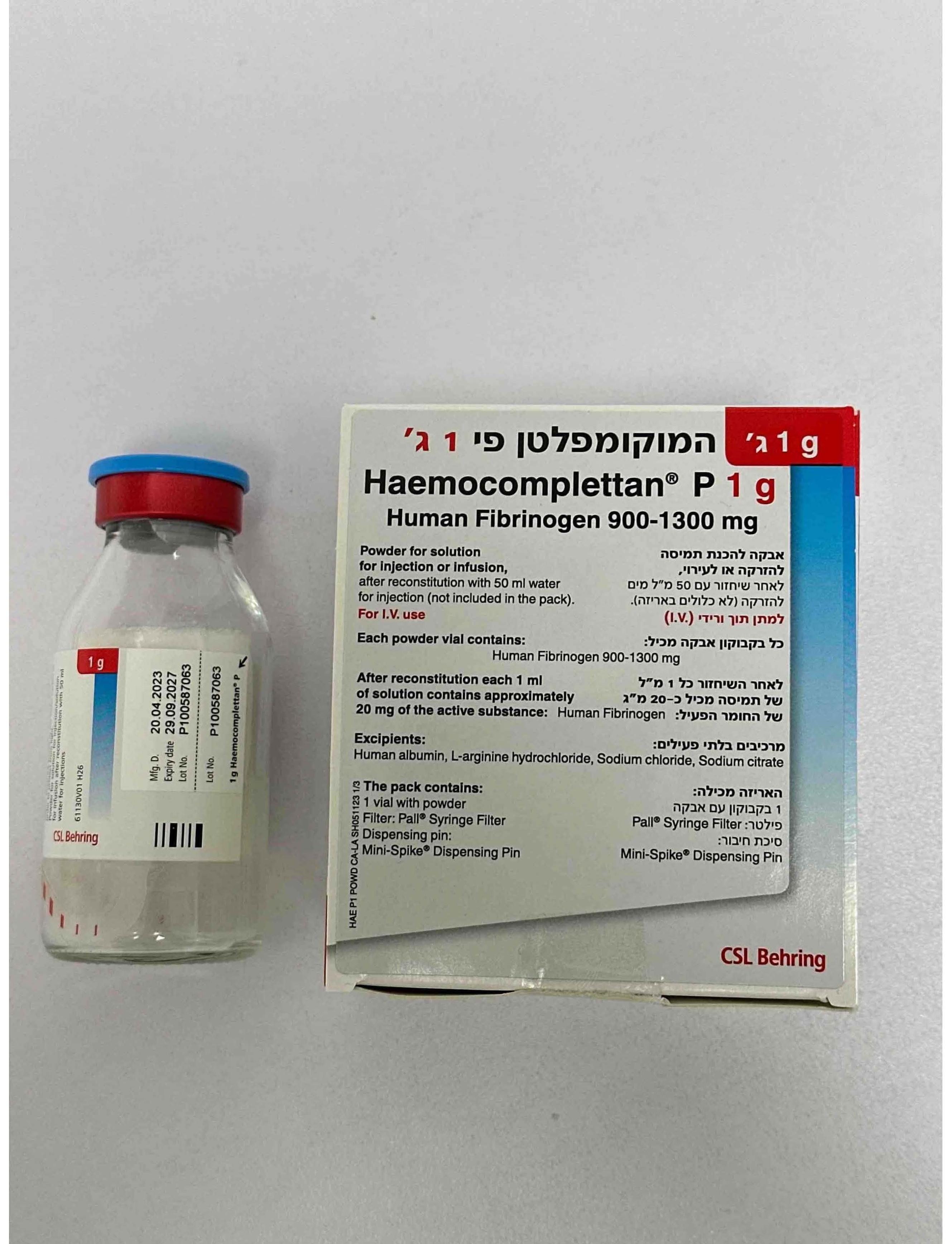
המוקומפלטן פי 1 ג' HAEMOCOMPLETTAN P 1 G (HUMAN FIBRINOGEN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להמסה להזרקהאינפוזיה : POWDER FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Treatment should be initiated under the supervision of a physician experienced in the treatment of coagulation disorders. Posology The dosage and duration of the substitution therapy depend on the severity of the disorder, location and extent of bleeding and the patient’s clinical condition. The (functional) fibrinogen level should be determined in order to calculate individual dosage and the amount and frequency of administration should be determined on an individual patient basis by regular measurement of plasma fibrinogen level and continuous monitoring of the clinical condition of the patient and other replacement therapies used. Normal plasma fibrinogen level is in the range of 1.5 – 4.5 g/l. The critical plasma fibrinogen level below which haemorrhages may occur is approximately 0.5 – 1.0 g/l. In case of major surgical intervention, precise monitoring of replacement therapy by coagulation assays is essential. 1. Prophylaxis in patients with congenital hypo- or afibrinogenaemia and known bleeding tendency. To prevent excessive bleeding during surgical procedures, prophylactic treatment is recommended to raise fibrinogen levels to 1 g/l and maintain fibrinogen at this level until haemostasis is secure and above 0.5 g/l until wound healing is complete. Initial Dose If the patient's fibrinogen level is not known, the recommended dose is 70 mg per kg of body weight (bw) administered intravenously. Subsequent Dose The target level (1 g/l) for minor events (e.g. epistaxis, intramuscular bleeding or menorrhagia) should be maintained for at least three days. The target level (1.5 g/l) for major events (e.g. head trauma or intracranial haemorrhage) should be maintained for seven days. Dose of fibrinogen (mg/kg b.w.) = [Target level (g/L) - measured level (g/L)] 0.017 (g/L per mg/kg b.w.) Furthermore, the amount to be administered and the frequency of application of Haemocomplettan P 1g/2g should always be oriented to the degree of bleeding and the clinical efficacy in the individual case. In case of major surgical intervention, precise monitoring of replacement therapy by coagulation assays is essential. Treatment of bleeding Adults Generally 1-2 g is administered initially with subsequent infusions as required. In case of severe haemorrhage i.e. obstetric use / abruption placenta, large amounts (4-8 g) of fibrinogen may be required. Children Limited data from clinical studies regarding the dosage of Haemocomplettan P1 g/2g in children are available. The dosage should be determined according to the body weight and clinical need but is usually 20-30 mg/kg. Method of Administration Intravenous infusion or injection. Haemocomplettan should be reconstituted according to section 6.6. The reconstituted solution should be warmed to room or body temperature before administration., then injected or infused slowly at a rate which the patient finds comfortable. The injection or infusion rate should not exceed approx. 5 ml per minute.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
