Quest for the right Drug
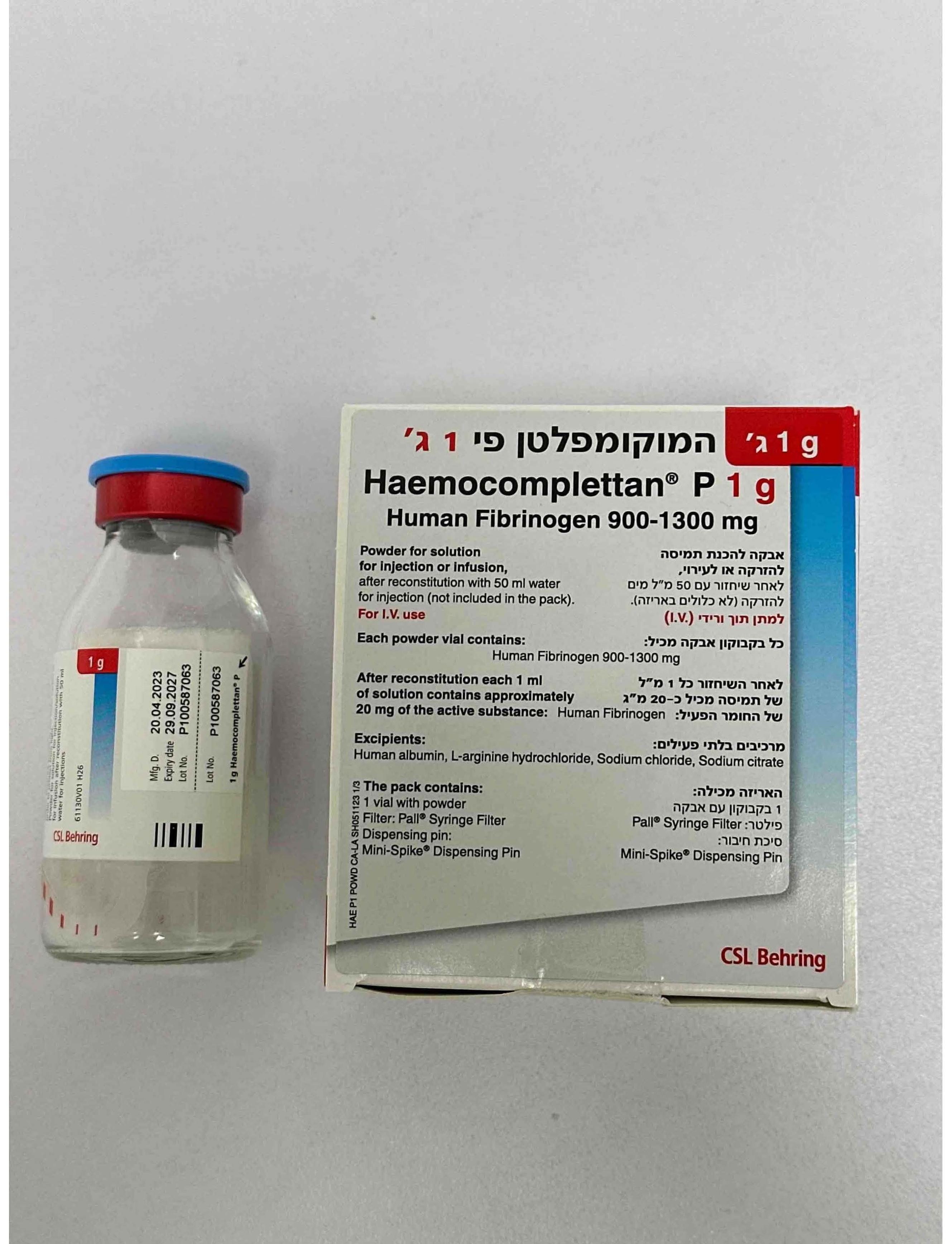
המוקומפלטן פי 1 ג' HAEMOCOMPLETTAN P 1 G (HUMAN FIBRINOGEN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להמסה להזרקהאינפוזיה : POWDER FOR SOLUTION FOR INJ/INF
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use There is a risk of thrombosis when patients with congenital deficiency are treated with human fibrinogen concentrate, particularly with high dose or repeated dosing. Patients given human fibrinogen concentrate should be observed closely for signs or symptoms of thrombosis. In patients with a history of coronary heart disease or myocardial infarction, in patients with liver disease, in peri- or post-operative patients, in neonates, or in patients at risk of thromboembolic events or disseminated intravascular coagulation, the potential benefit of treatment with human fibrinogen concentrate should be weighed against the risk of thromboembolic complications. Caution and close monitoring should also be performed. If allergic or anaphylactic-type reactions occur, the injection/infusion should be stopped immediately. In case of anaphylactic shock, standard medical treatment for shock should be implemented. In the case of replacement therapy with coagulation factors in other congenital deficiencies, antibody reactions have been observed, but there is currently no data available with regard to fibrinogen. Important information about specific excipients of Haemocomplettan Haemocomplettan contains up to 164 mg (7.1 mmol) sodium per 1g fibrinogen. This correlates with 11.5 mg (0.5 mmol) sodium per kg body weight of the patient if the recommended initial dose of 70 mg/kg body weight is applied. To be taken into consideration by patients on a controlled sodium diet. Virus safety Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infective agents cannot be totally excluded. This also applies to unknown or emerging viruses and other pathogens. The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) and for the non-enveloped hepatitis A virus (HAV). The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19. Parvovirus B19 infections may be serious for pregnant women (foetal infection) and for individuals with immunodeficiency or increased erythropoiesis (e.g. haemolytic anaemia). Appropriate vaccination (hepatitis A and hepatitis B) should be considered for patients in regular/repeated receipt of products from human blood or plasma. It is strongly recommended that every time that Haemocomplettan is administered to a patient, the name and batch number of the product are recorded in order to maintain a link between the patient and the batch of the product.
Effects on Driving
4.7 Effects on ability to drive and use machines Haemocomplettan has no influence on the ability to drive and use machines.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
