Quest for the right Drug
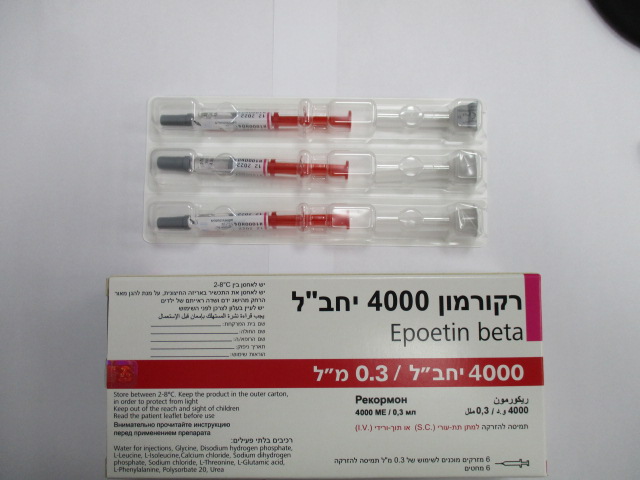
רקורמון 4000 IU RECORMON 4000 IU (EPOETIN BETA)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי, תת-עורי : I.V, S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: antianemic, ATC code: B03XA01 Mechanism of action Erythropoietin is a glycoprotein that stimulates the formation of erythrocytes from its committed progenitors. It acts as a mitosis stimulating factor and differentiation hormone. Epoetin beta, the active substance of Recormon is identical in its amino acid and carbohydrate composition to erythropoietin that has been isolated from the urine of anaemic patients. The biological efficacy of epoetin beta has been demonstrated after intravenous and subcutaneous administration in various animal models in vivo (normal and uraemic rats, polycythaemic mice, dogs). After administration of epoetin beta, the number of erythrocytes, the Hb values and reticulocyte counts increase as well as the 59 Fe-incorporation rate. 3 An increased H-thymidine incorporation in the erythroid nucleated spleen cells has been found in vitro (mouse spleen cell culture) after incubation with epoetin beta. Investigations in cell cultures of human bone marrow cells showed that epoetin beta stimulates erythropoiesis specifically and does not affect leucopoiesis. Cytotoxic actions of epoetin beta on bone marrow or on human skin cells were not detected. After single dose administration of epoetin beta no effects on behaviour or locomotor activity of mice and circulatory or respiratory function of dogs were observed. Clinical efficacy and safety In a randomised, double-blind, placebo-controlled study of 4,038 chronic renal failure patients not on dialysis with type 2 diabetes and haemoglobin levels ≤ 11 g/dL, patients received either treatment with darbepoetin alfa to target haemoglobin levels of 13 g/dL or placebo (see section 4.4). The study did not meet either primary objective of demonstrating a reduction in risk for all-cause mortality, cardiovascular morbidity, or end stage renal disease (ESRD). Analysis of the individual components of the composite endpoints showed the following HR (95% CI): death 1.05 (0.92, 1.21), stroke 1.92 (1.38, 2.68), congestive heart failure (CHF) 0.89 (0.74, 1.08), myocardial infarction (MI) 0.96 (0.75, 1.23), hospitalisation for myocardial ischaemia 0.84 (0.55, 1.27), ESRD 1.02 (0.87, 1.18). Pooled post-hoc analyses of clinical studies with ESAs have been performed in CRF patients (on dialysis, not on dialysis, with or without diabetes). A tendency towards increased risk estimates for all-cause mortality, cardiovascular and cerebrovascular events associated with higher cumulative ESA doses independent of the diabetes or dialysis status was observed (see sections 4.2 and 4.4). Erythropoietin is a growth factor that primarily stimulates red cell production. Erythropoietin receptors may be expressed on the surface of a variety of tumour cells. Survival and tumour progression have been examined in five large controlled studies involving a total of 2833 patients, of which four were double-blind placebo-controlled studies and one was an open-label study. Two of the studies recruited patients who were being treated with chemotherapy. The target haemoglobin concentration in two studies was >13 g/dl; in the remaining three studies it was 12-14 g/dl. In the open-label study there was no difference in overall survival between patients treated with recombinant human erythropoietin and controls. In the four placebo-controlled studies the hazard ratios for overall survival ranged between 1.25 and 2.47 in favour of controls. These studies have shown a consistent unexplained statistically significant excess mortality in patients who have anemia associated with various common cancers who received recombinant human erythropoietin compared to controls. Overall survival outcome in the trials could not be satisfactorily explained by differences in the incidence of thrombosis and related complications between those given recombinant human erythropoietin and those in the control group. An individual patient data based meta-analysis, which included data from all 12 controlled clinical studies in anaemic cancer patients conducted with Recormon (n=2301), showed an overall hazard ratio point estimate for survival of 1.13 in favour of controls (95 % CI 0.87, 1.46). In patients with baseline haemoglobin ≤ 10 g/dl (n=899), the hazard ratio point estimate for survival was 0.98 (95 % CI 0.68 to 1.40). An increased relative risk for thromboembolic events was observed in the overall population (RR 1.62, 95 % CI: 1.13, 2.31). A patient-level data analysis has also been performed on more than 13,900 cancer patients (chemo-, radio-, chemoradio- or no therapy) participating in 53 controlled clinical trials involving several epoetins. Meta-analysis of overall survival data produced a hazard ratio point estimate of 1.06 in favour of controls (95% CI: 1.00, 1.12; 53 trials and 13,933 patients) and for cancer patients receiving chemotherapy, the overall survival hazard ratio was 1.04 (95% CI: 0.97, 1.11; 38 trials and 10, 441 patients). Meta-analyses also indicate consistently a significantly increased relative risk of thromboembolic events in cancer patients receiving recombinant human erythropoietin (see section 4.4). In very rare cases, neutralising anti-erythropoietin antibodies with or without pure red cell aplasia (PRCA) occurred during rHuEPO therapy. Premature infants In premature infants there may be a slight rise in platelet counts, particularly up to day 12 - 14 of life, therefore platelets should be monitored regularly. In preterm infants a potential risk of erythropoietin to cause retinopathy could not be excluded, therefore caution should be exercised and the decision to treat a preterm infant should be balanced against the potential benefit and risk of this treatment and available alternative options. In premature infants, a fall in serum ferritin values is very common.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Pharmacokinetic investigations in healthy volunteers and uraemic patients show that the half-life of intravenously administered epoetin beta is between 4 and 12 hours and that the distribution volume corresponds to one to two times the plasma volume. Analogous results have been found in animal experiments in uraemic and normal rats. After subcutaneous administration of epoetin beta to uraemic patients, the protracted absorption results in a serum concentration plateau, whereby the maximum concentration is reached after an average of 12 - 28 hours. The terminal half-life is higher than after intravenous administration, with an average of 13 - 28 hours. Bioavailability of epoetin beta after subcutaneous administration is between 23 and 42 % as compared with intravenous administration.

פרטי מסגרת הכללה בסל
התרופה תינתן בכל אחד מאלה: 1. אנמיה חמורה (severe anemia) בחולי אי ספיקה כלייתית כרונית. 2. חולים אנמיים הסובלים ממחלה ממאירה והמקבלים טיפול פעיל ייעודי במחלתם וכן לחולים הסובלים ממיאלומה נפוצה (multiple myeloma) או מהתסמונת המיאלודיספלסטית (myelodisplastic syndrome) שנתקיימו בהם כל אלה: 1. אחד מהתנאים האלה: א. רמת המוגלובין נמוכה מ-8 גרם %. ב. החולה מרותק למיטתו בגלל אנמיה המלווה במחלת לב איסכמית או באי ספיקה לבבית. ג. החולה נזקק לקבלת שתי מנות דם לפחות פעם בשבועיים במשך חודשיים. 2. נשללה סיבה אחרת לאנמיה שאינה קשורה לטיפול הייעודי במחלתם האמורה לעיל ובכלל זה דימום, חוסר ברזל, חוסר חומצה פולית, חוסר ויטמין B12 והמוליזה. 3. רמת אריתרופואטין בנסיוב נמוכה מ-100 mu/ml.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| EPOETIN THETA (R-HUEPO) | ||||
| EPOETIN ALFA | ||||
| EPOETIN BETA | ||||
| DARBEPOETIN ALFA | ||||
| חולים אנמיים הסובלים ממחלה ממאירה והמקבלים טיפול פעיל ייעודי במחלתם וכן לחולים הסובלים ממיאלומה נפוצה (multiple myeloma) או מהתסמונת המיאלודיספלסטית (myelodisplastic syndrome | ||||
| אנמיה חמורה (severe anemia) בחולי אי ספיקה כלייתית כרונית. | ||||
| oncology | ||||
| CKD |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2002
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
