Quest for the right Drug
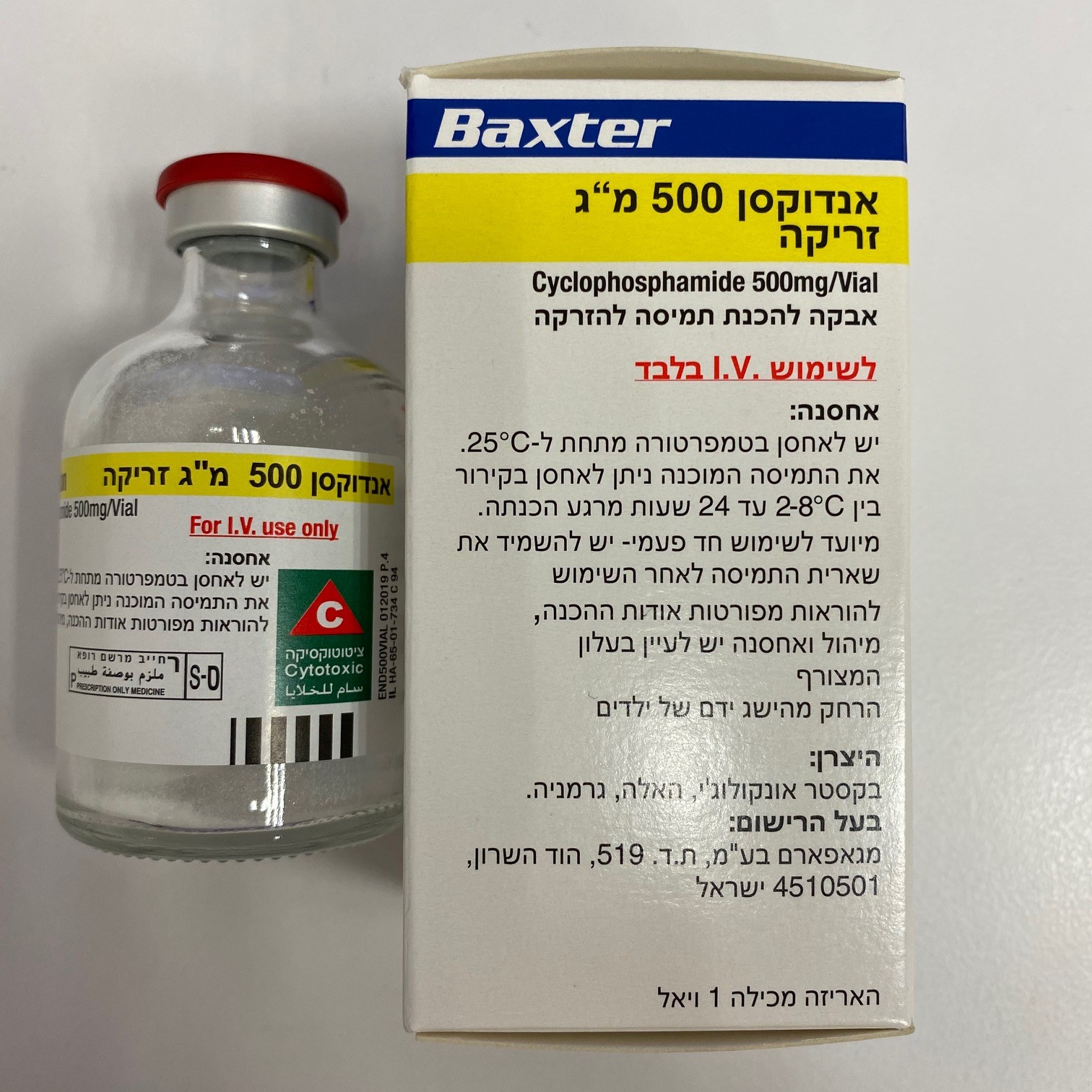
אנדוקסן 500 מ"ג זריקה ENDOXAN 500 MG INJECTION (CYCLOPHOSPHAMIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להכנת תמיסה לזריקה : POWDER FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The following adverse drug reactions are based on post marketing data. They are listed in the table according to MedDRA system organ class and by frequency. Side effect frequencies are based on the following categories: Very common: Common: ( 1/10) ( 1/100 to < 1/10) Uncommon: Rare: ( 1/1,000 to < 1/100) ( 1/10,000 to < 1/1,000) Very rare: (< 1/10,000) Unknown: Unknown (adverse reactions reported in the post-marketing experience) Adverse drug reactions System organ class Side effect Frequency Infections and Infections1 Common infestations Pneumonias2 Uncommon Sepsis1 Uncommon Septic shock Unknown Adverse drug reactions System organ class Side effect Frequency Neoplasms benign, Secondary tumors Rare malignant and Acute leukemia3 Rare unspecified (including Myelodysplastic syndrome Rare incl cysts and polyps) Bladder cancer Rare Tumor lysis syndrome Unknown Blood and lymphatic Myelosuppression4 Very common system disorders Disseminated intravascular coagulation (DIC) Very rare Hemolytic uremic syndrome Very common Lymphopenia Unknown Immune system Immunosuppression Very common disorders Hypersensitivity reactions Uncommon Anaphylactic/anaphylactoid reaction Very rare Endocrine disorders Syndrome of inadequate ADH secretion Rare (SIADH) Metabolism and Anorexia Uncommon nutrition disorders Dehydration Rare Hyponatremia Very rare Fluid retention Very rare Changes in blood glucose level (increase or Unknown decrease) Psychiatric diseases Confusion Very rare Nervous system Dizziness Rare disorders Convulsion Very rare Encephalopathy Unknown Neurotoxicity5 Unknown Eye disorders Visual impairment Rare Conjunctivitis Very rare Eye edema Very rare Lacrimation increased Unknown Ear and labyrinth Deafness Unknown disorders Tinnitus Unknown Cardiac disorders Cardiomyopathy Unknown Myocarditis Unknown Cardiac failure (including isolated fatal cases) Unknown Arrhythmias6 Unknown Myocardial infarction Unknown Adverse drug reactions System organ class Side effect Frequency Pericarditis Unknown Cardiogenic shock Unknown Pericardial effusion Unknown Electrocardiogram QT interval prolonged Unknown Ventricular fibrillation Unknown Ventricular tachycardia Unknown Vascular disorders Flushing Uncommon Pulmonary embolism Unknown Venous thrombosis Unknown Vasculitis Unknown Peripheral ischemia Unknown Respiratory, thoracic Acute respiratory distress syndrome (ARDS) Unknown and mediastinal Pulmonary edema Unknown disorders Pulmonary hypertension Unknown Bronchospasm Unknown Dyspnea Unknown Hypoxia Unknown Cough Unknown Nasal congestion Unknown Rhinorrhea Unknown Pulmonary veno-occlusive disease Unknown Interstitial Lung Diseases7 Unknown Oropharyngeal pain Unknown Gastrointestinal Stomatitis Very rare disorders Diarrhea Very rare Vomiting Very rare Constipation Very rare Nausea Very rare Hemorrhagic enterocolitis Very rare Acute pancreatitis Very rare Mucosal ulceration Very rare Gastrointestinal hemorrhage Unknown Abdominal pain Unknown Parotid gland inflammation Unknown Colitis Unknown Enteritis Unknown Cecitis Unknown Adverse drug reactions System organ class Side effect Frequency Hepatobiliary disorders Hepatic function abnormal Common Veno-occlusive disorder Unknown Hepatitis Unknown Cholestasis Unknown Hepatotoxicity8 Unknown Skin and subcutaneous Alopecia Very common tissue disorders Dermatitis Rare Discoloration of palms, fingernails, and soles Rare of the feet Stevens Johnson syndrome (SJS) Very rare Toxic epidermal necrolysis (TEN) Very rare Erythema multiforme Unknown Radiation recall dermatitis Unknown Pruritus (including inflammatory itching) Unknown Palmar-plantar erythrodysesthesia Unknown Urticaria Unknown Blistering Unknown Facial swelling Unknown Hyperhidrosis Unknown Rash Rare Erythema in irradiated area Unknown Erythema Unknown Musculoskeletal and Rhabdomyolysis Unknown connective tissue Scleroderma Unknown disorders Muscle spasms Unknown Myalgia Unknown Arthralgia Unknown Renal and urinary Cystitis Very common disorders Microhematuria Very common Hemorrhagic cystitis (including isolated fatal Common cases) Macrohematuria Common Suburethral bleeding Very rare Edema of the bladder wall Very rare Interstitial inflammation with fibrosis and Very rare sclerosis of the bladder Renal failure Very rare Blood creatinine increased Very rare Adverse drug reactions System organ class Side effect Frequency Renal Tubular necrosis Unknown Renal tubular disorder Unknown Toxic nephropathy Unknown Hemorrhagic ureteritis Unknown Ulcerative cystitis Unknown Bladder contracture Unknown Nephrogenic diabetes insipidus Unknown Atypical bladder epithelial cells Unknown Blood urea nitrogen increased Unknown Pregnancy, puerperium Premature labor Unknown and perinatal conditions Reproductive system Impairment of spermatogenesis Common and breast disorders Ovulation disorder Uncommon Amenorrhea9 Rare Azoospermia9 Rare Oligospermia9 Rare Infertility Unknown Ovarian failure Unknown Oligomenorrhea Unknown Testicular atrophy Unknown Blood estrogen decreased Unknown Blood gonadotropin increased Unknown Congenital, familial and Intra-uterine death of the fetus Unknown genetic disorders Fetal malformation Unknown Fetal growth retardation Unknown Fetal toxicity (including Unknown myelosuppression/gastroenteritis) General disorders and Fever Very common administration site Asthenia Common conditions Mucosal inflammation Common Chest pain Rare Headache Very rare Multi-organ failure Unknown Edema Unknown Flu-like illness Unknown General physical deterioration Unknown Injection/infusion site reactions10 Unknown Adverse drug reactions System organ class Side effect Frequency Investigations Blood lactate dehydrogenase increased Unknown C-reactive protein increased Unknown 1 including other bacterial, fungal, viral, protozoal, parasitic, reactivation of latent infections, including viral hepatitis, tuberculosis, JC virus with progressive multifocal leukoencephalopathy (including fatal outcomes), Pneumocystis jiroveci, herpes zoster, Strongyloides. 2 including fatal outcomes. 3 including acute myeloid leukemia and acute promyelocytic leukemia. 4 manifested as Bone marrow failure, Pancytopenia, Neutropenia, Agranulocytosis, Granulocytopenia, Thrombocytopenia (complicated by bleeding), Leukopenia, Anemia 5 manifested as reversible posterior leukoencephalopathy syndrome, myelopathy, peripheral neuropathy, polyneuropathy, neuralgia, dysesthesia, hypoesthesia, paresthesia, tremor, dysgeusia, hypogeusia, parosmia. 6 manifested as Atrial fibrillation, Supraventricular arrhythmia, Ventricular arrhythmia, Bradycardia, Tachycardia, Palpitation 7 manifested by pulmonary fibrosis, obliterative bronchiolitis, organizing pneumonia, alveolitis allergic, pneumonitis. 8 Hepatic failure, Hepatic encephalopathy, Ascites, Hepatomegaly, Jaundice, Blood bilirubin increased, Hepatic enzymes increased (ASAT, ALAT, ALP, gamma-GT) 9 persistent 10 manifested by thrombosis, necrosis, phlebitis, inflammation, pain, swelling, erythema. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
Hodgkin's disease, malignant lymphomas, multiple myeloma, mycosis fungoides, neuroblastoma, autoimmune disease
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
