Quest for the right Drug
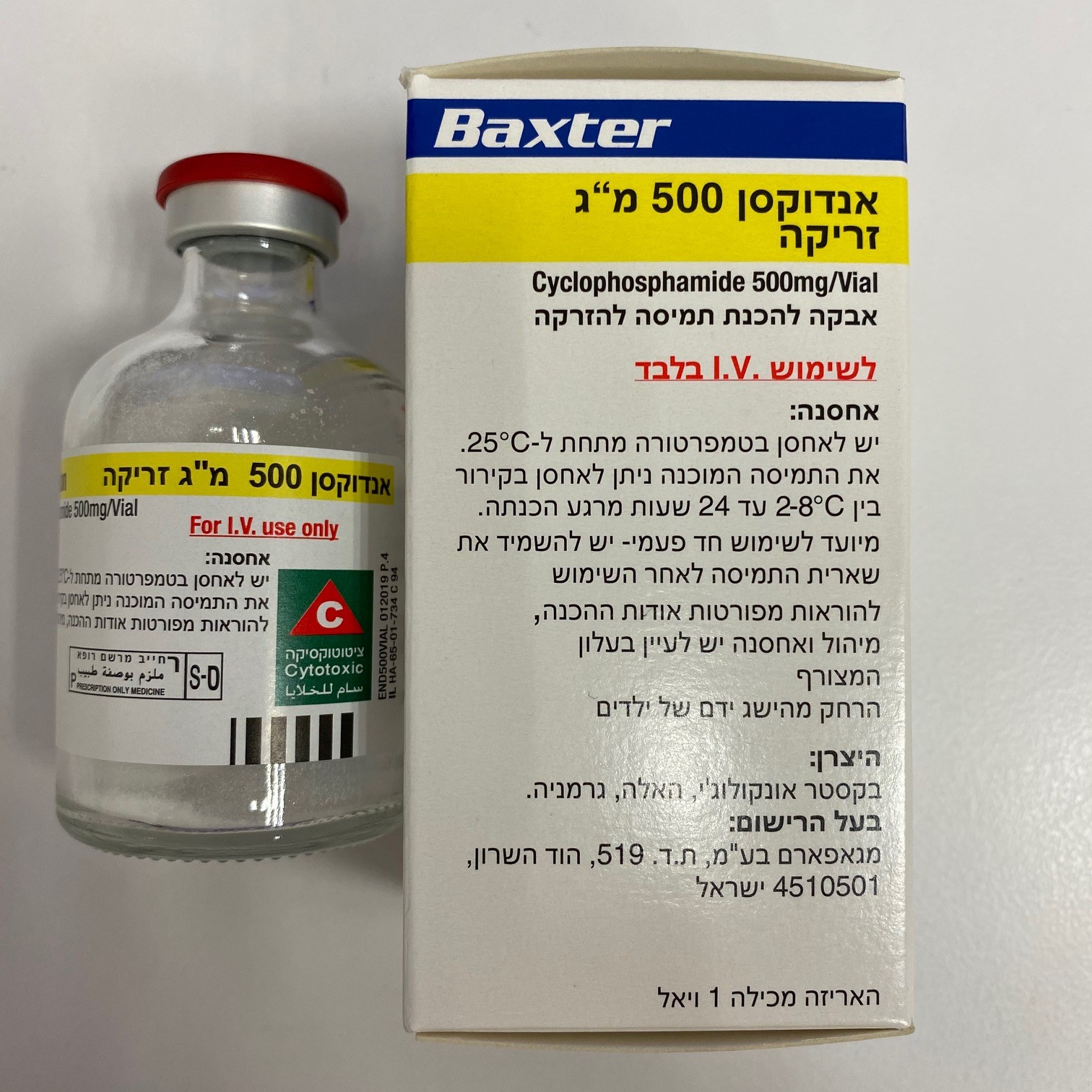
אנדוקסן 500 מ"ג זריקה ENDOXAN 500 MG INJECTION (CYCLOPHOSPHAMIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להכנת תמיסה לזריקה : POWDER FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Treatment of lupus nephritis with Endoxan should only be performed by physicians who have specific experience with the diseases and with Endoxan. Posology Endoxan should only be administered by physicians experienced with this drug. The dosage must be adapted to each patient individually. The following dose recommendations mainly apply to the treatment with cyclophosphamide as a monotherapy. In combination with other cytostatics of similar toxicity a dose reduction or extension of the therapy-free intervals may be necessary. Attention should be paid to adequate hydration as well as to the administration of the Uroprotector and Uromitexan. The handling and preparation of cytostatics should always be in accordance with the safety precautions used for the handling of cytotoxic agents. Unless otherwise prescribed the following dosages are recommended: Endoxan 500 mg / 1 g • for continuous treatment in adults and children 3 to 6 mg/kg body weight daily (equivalent to 120 to 240 mg /m2 body surface). • for intermittent treatment 10 to 15 mg/kg body weight (equivalent to 400 to 600 mg /m2 body surface) at intervals of 2 to 5 days. • for high-dose intermittent treatment, e.g. 20 to 40 mg/kg body weight (equivalent to 800 to 1600 mg/m2 body surface) and higher doses (e.g. for conditioning prior to bone- marrow transplantation) at intervals of 21 to 28 days. Patients with hepatic impairment Severe hepatic impairment may be associated with decreased activation of cyclophosphamide. This may alter the effectiveness of Endoxan treatment and should be considered when selecting the dose and interpreting the response to the dose selected. Patients with renal impairment In patients with renal impairment, particularly in patients with severe renal impairment, decreased renal excretion may result in increased plasma levels of cyclophosphamide and its metabolites. This may result in increased toxicity and should be considered when determining the dosage in such patients. In the presence of renal impairment, dose reduction by 50% is a common recommendation for glomerular filtration rates below 10 mL per minute. Cyclophosphamide and its metabolites are dialyzable, although there may be differences in clearance depending on the dialysis system being used. In patients requiring dialysis, the time between Endoxan administration and dialysis should be consistent (see section 4.4). Recommendations for dose reduction in the presence of myelosuppression WBC count [L] Platelet count [L] 4,000 100,000 100% of the proposed dose 4,000-2,500 100,000 to 50,000 50% of the proposed dose 2,500 50,000 Postponement until normalization or individual decision Geriatric population In elderly patients, monitoring for toxicities and the need for dose adjustment should reflect the higher frequency of decreased hepatic, renal, cardiac, or other organ function, and concomitant diseases or other drug therapy in this population. Method of administration Cyclophosphamide is inert until activated by enzymes in the liver. However, as with all cytotoxics, it is suggested that reconstitution should be performed by trained personnel, in a designated area. Those handling the preparation should wear protective gloves. Care should be taken to avoid splashing material into the eyes. The material should not be handled by women who are pregnant or who are breast-feeding. Intravenous administration Intravenous administration preferably should be conducted as an infusion, usually given directly into the tubing of a fast running I.V. infusion with the patient supine. Care should be taken that extravasation does not take place, however, should it occur, no specific measures need be taken. Duration of the infusion also should be appropriate for the volume and type of carrier fluid to be infused. If injected directly, cyclophosphamide for parenteral administration should be reconstituted with physiological saline (0.9% sodium chloride). The pH of an aqueous solution is between 4 and 6. Cyclophosphamide, reconstituted in water, is hypotonic and should not be injected directly. For infusion, cyclophosphamide should be reconstituted by adding sterile water and infused in the recommended intravenous solutions. Before parenteral administration, the substance must be completely dissolved. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Notes on preparation and handling of the solution To prepare a 2% isotonic solution, add the corresponding amount of physiological saline solution to the dry substance (25 mL to Endoxan 500 mg and 50 mL to Endoxan 1 g). The substance dissolves readily when the vial is shaken vigorously after injecting the solvent. Allow the solution to stand for several minutes, if necessary. For i tra e ous short i fusio , add e.g. Ri ger’s solutio , sali e solutio or glu ose solutio to the Endoxan solution to achieve a volume of 500 ml.

שימוש לפי פנקס קופ''ח כללית 1994
Hodgkin's disease, malignant lymphomas, multiple myeloma, mycosis fungoides, neuroblastoma, autoimmune disease
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
