Quest for the right Drug
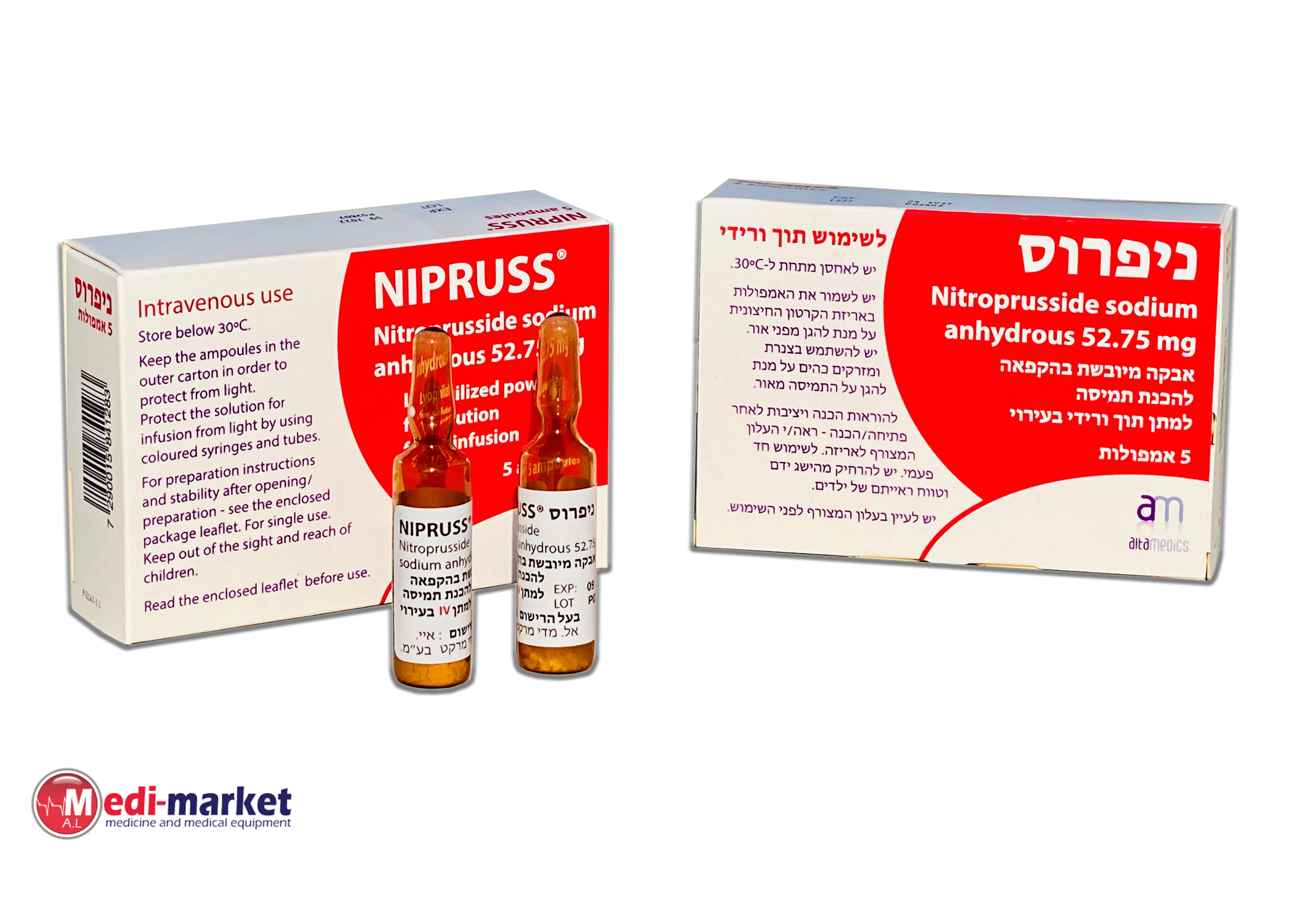
ניפרוס NIPRUSS ® (NITROPRUSSIDE SODIUM ANHYDROUS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה להכנת תמיסה לאינפוזיה : POWDER FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects During administration of sodium nitroprusside, the following undesirable effects may be observed. The undesirable effects are listed below according to system organ class (SOC). Within each system organ class they are ranked by frequency starting with the most common. Within each frequency group, the undesired effects are listed in order of decreasing severity. In addition, the appropriate frequency category for each side effect is based on the following frequency definitions: Very common (≥ 1/10) Common (≥1/100 to < 1/10) Uncommon (≥1/1.000 to < 1/100) Rare (≥ 1/10.000 to < 1/1.000) Very rare (< 1/10.000) Not known (cannot be estimated from the available data). Blood and lymphatic system disorders Frequency not known: Bright red venous blood Metabolism and nutrition disorders Frequency not known: Metabolic acidosis, lactate increase, loss of appetite, hypothyroidism Psychiatric disorders Frequency not known: Psychosis Nervous system disorders Frequency not known: Headache, dizziness, sleep disorders, nervousness, tinnitus, miosis, hyperreflexia, confusion, hallucinations, seizures, paralysis, coma Cardiac disorders Frequency not known: Tachycardia, cardiac arrhythmia, palpitations Vascular disorders Frequency not known: Severe hypotension, rebound effect Respiratory, thoracic and mediastinal disorders Frequency not known: Hypoventilation, decreased oxygen uptake, respiratory paralysis Gastrointestinal disorders Frequency not known: Vomiting, nausea, diarrhoea, incontinence General disorders and administration site conditions Frequency not known: Weakness, insufficient lowering of blood pressure, tachyphylaxis, tolerance (more likely in younger patients than in elderly); infusion site reactions (e.g.: pain, reddening of the skin, itching) Injury, poisoning and procedural complications Frequency not known: Cyanide intoxication, thiocyanate intoxication Description of selected adverse reactions: Insufficient blood pressure reduction and the occurrence of tachyphylaxis and/or tolerance are to be expected in younger rather than older hypertension patients. Symptoms of cyanide intoxication Cyanide toxicity may manifest as bright red venous blood, hypoventilation, increased lactate, decreased oxygen uptake, palpitations, cardiac arrhythmias, headache, metabolic acidosis, coma, respiratory paralysis and seizures. Deaths have been reported. Such signs of toxicity can occur if the dose of 0.05 mg CN-/kg/min, which corresponds to the detoxification capacity of the human body, is exceeded without a simultaneous administration of thiosulfate. Cyanide intoxication is completely avoidable by simultaneously administering a thiosulfate infusion at a molar ratio of 5 : 1 (thiosulfate : sodium nitroprusside). Symptoms of thiocyanate intoxication Cyanide together with thiosulfate is metabolised to thiocyanate, which is approximately 100 times less toxic compared to cyanide. However, it should be taken into account that thiocyanate is only eliminated slowly and can therefore lead to severe intoxication if sodium nitroprusside is infused for a long time. Symptoms of thiocyanate toxicity that can occur in case of overdose – earlier in renally impaired patients than in renally healthy patients – include: dizziness, headache, loss of appetite, sleep disorders, nervousness, hypothyroidism, diarrhoea, vomiting, incontinence, psychosis, paralysis and coma. Very high serum concentrations can lead to death. The symptoms of thiocyanate intoxication are also avoidable when the dosing instructions are observed. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form /https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
