Quest for the right Drug
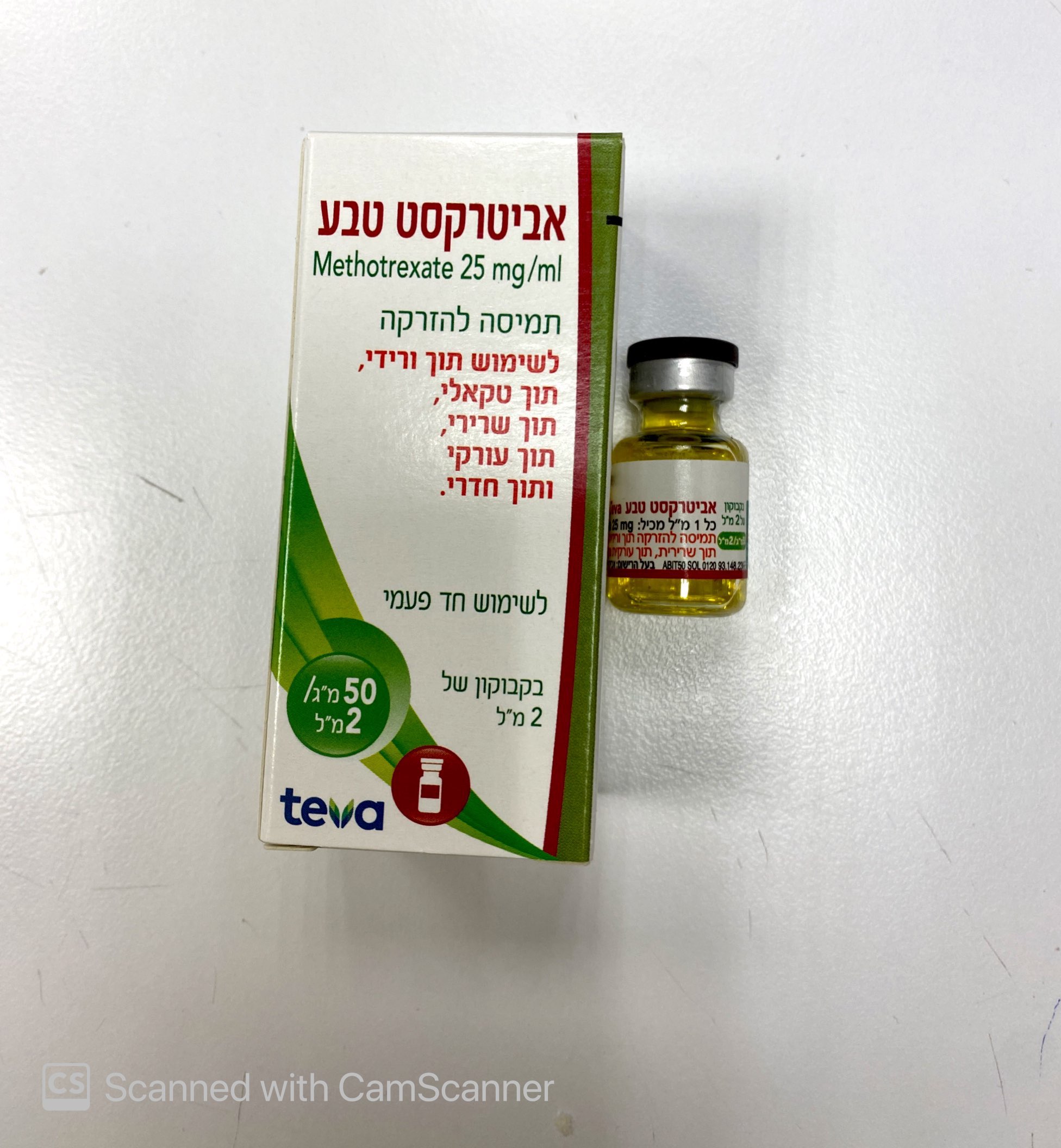
אביטרקסט טבע ABITREXATE TEVA (METHOTREXATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי, תוך-שדרתי, תוך-עורקי, תוך חדרי : I.M, I.V, INTRATHECAL, INTRA-ARTERIAL, INTRA VENTRICULAR
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
ADVERSE REACTIONS Adverse Drug Reaction Overview In general, the incidence and severity of acute side effects are related to dose, frequency of administration, and the duration of the exposure to significant blood levels of methotrexate to the target organs. The most serious reactions are discussed under WARNINGS AND PRECAUTIONS section. The most frequently reported adverse reactions include ulcerative stomatitis, leucopenia, nausea, and abdominal distress. Other frequently reported adverse effects are malaise, undue fatigue, chills and fever, dizziness and decreased resistance to infection. Ulcerations of the oral mucosa are usually the earliest signs of toxicity. Adverse Drug Reactions by Organ System Blood and lymphatic Leucopenia, anemia, aplastic anemia, thrombopenia, system disorders: pancytopenia, agranulocytosis, lymphadenopathy and lymphoproliferative disorders (including reversible), neutropenia and eosinophilia have also been observed. Cardiac disorders: Pericarditis and pericardial effusion (damage to heart, rarely). Eye disorders: Conjunctivitis, blurred vision, serious visual changes of unknown etiology, and transient blindness/vision loss. Gastrointestinal Gingivitis, stomatitis, enteritis, anorexia, nausea, disorders: vomiting, diarrhea, hematemesis, melena, gastrointestinal ulceration and bleeding, pancreatitis, intestinal perforation, non-infectious peritonitis, glossitis. General disorders and Anaphylactoid reactions, vasculitis, fever, administration site conjunctivitis, infection, sepsis, nodulosis, conditions: hypogammaglobulinaemia, and sudden death. Hepatobiliary disorders: Hepatotoxicity, acute hepatitis, chronic fibrosis and cirrhosis, decrease in serum albumin, liver enzyme elevations, hepatic failure. Infection: Other reported infections included nocardiosis, histoplasmosis, cryptococcosis, and disseminated H. simplex, cytomegalovirus infection, including cytomegaloviral pneumonia. Metabolism and Diabetes mellitus. nutrition disorders: Musculoskeletal, Stress fractures, soft tissue necrosis, osteonecrosis, connective tissue arthralgia, myalgia and osteoporosis. and bone disorders: Neoplasms benign, malignant and unspecified Tumour lysis syndrome. Malignant lymphomas, (including cysts and polyps): which may regress following withdrawal of methotrexate, may occur in patients receiving low- dose methotrexate, and thus may not require cytotoxic treatment. Discontinue methotrexate first and, if the lymphoma does not regress, appropriate treatment should be instituted. Nervous system: Cerebrospinal fluid pressure increased, neurotoxicity, arachnoiditis, paresthesia, headache, dizziness, drowsiness, speech impediment including dysarthria and aphasia; hemiparesis, paresis and convulsions. Following low doses, there have been occasional reports of transient subtle cognitive dysfunction, mood alteration, or unusual cranial sensations, leukoencephalopathy, or encephalopathy. Renal and urinary Renal failure, severe nephropathy or renal failure, disorders: azotemia, dysuria, cystitis, hematuria, urogenital dysfunction. Proteinuria has also been observed. Reproductive system Defective oogenesis or spermatogenesis, transient and breast disorders: oligospermia, menstrual dysfunction, vaginal discharge and gynecomastia; infertility, abortion, fetal defects, loss of libido/impotence. Respiratory, thoracic Pneumonia, interstitial alveolitis/pneumonitis often and mediastinal disorders: associated with eosinophilia, pulmonary fibrosis, Pneumocystis carinii pneumonia, pleural effusion. Dyspnea, chest pain, hypoxia, respiratory fibrosis, pharyngitis, and chronic interstitial obstructive pulmonary disease, alveolitis and pulmonary alveolar haemorrhage have occasionally occurred. Skin disorders: Erythema, pruritus, photosensitisation, petechiae, loss of hair, skin necrosis, exfoliative dermatitis, painful erosion of psoriatic plaques, herpes zoster, vasculitis, urticaria, pigmentary changes, acne, ecchymosis, Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell’s syndrome), furunculosis and telangiectasia. Drug reaction with eosinophilia and systemic symptoms Vascular disorders: Hypotension, and thromboembolic events (including arterial thrombosis, cerebral thrombosis, deep vein thrombosis, retinal vein thrombosis, thrombophlebitis, and pulmonary embolus), vasculitis Adverse Reactions Reported in Rheumatoid Arthritis: • Alopecia (common) • Diarrhea (common) • Dizziness (common) • Elevated liver enzymes (very common) • Leucopenia (common) • Nausea/vomiting (very common) • Pancytopenia (common) • Rash/pruritus/dermatitis (common) • Stomatitis (common) • Thrombocytopenia (common) Adverse Reactions in Psoriasis: The adverse reaction rates reported are very similar to those in the rheumatoid arthritis studies. Rarely, painful psoriatic plaque erosions may appear. Abnormal Hematologic and Clinical Chemistry Findings See WARNINGS AND PRECAUTIONS: Monitoring and Laboratory Tests section. Post-Market Adverse Drug Reactions Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse events have also been reported during post-marketing experience with methotrexate: System Organ Class Adverse Reaction Infections (including fatal sepsis); Pneumonia; Pneumocystis carinii pneumonia; Nocardiosis; Histoplasmosis; Cryptococcosis; Herpes zoster; Infections and Infestations H. simplex hepatitis; Disseminated H. simplex; Cytomegalovirus infection (including cytomegaloviral pneumonia); Reactivation of hepatitis B infection; Worsening of hepatitis C infection Agranulocytosis; Pancytopenia; Leukopenia; Neutropenia; Lymphadenopathy and lymphoproliferative Blood and Lymphatic System Disorders disorders (including reversible); Eosinophilia; Anemia megaloblastic; Renal vein thrombosis; Lymphoma; Aplastic anemia; Hypogammaglobulinemia CSF pressure increased; Neurotoxicity; Arachnoiditis; Nervous System Disorders Paraplegia; Stupor; Ataxia; Dementia; Dizziness; Paresthesia Chronic interstitial pulmonary disease; Respiratory, Thoracic and Mediastinal Disorders Alveolitis; Dyspnea; Chest pain; Hypoxia; Cough; Plural effusion Intestinal perforation; Noninfectious peritonitis; Gastrointestinal Disorders Glossitis; Nausea; Pancreatitis Hepatobiliary Disorders Hepatic failure Drug reaction with eosinophilia and systemic symptoms; Skin and Subcutaneous Tissue Disorders Dermatitis; Petechiae Musculoskeletal, Connective Tissue and Bone Disorders Osteonecrosis Renal and Urinary Disorders Proteinuria Pregnancy, Puerperium and Perinatal Conditions Fetal death, Abortion Reproductive System and Breast Disorders Urogenital dysfunction General Disorders and Administration Site Conditions Pyrexia; Chills; Malaise; Fatigue; Anaphylactic reactions Endocrine Disorders Diabetes Ophthalmologic Disorders Transient blindness/vision loss Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il. DRUG INTERACTIONS Serious Drug Interactions The use of nitrous oxide anesthesia with methotrexate is contraindicated (see CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS - Renal and DRUG INTERACTIONS - Drug-Drug Interactions). Overview Methotrexate competes with reduced folates for active transport across cell membranes by means of a single carrier-mediated active transport process. Impaired renal function, as well as concurrent use of drugs such as weak organic acids that undergo tubular secretion, can markedly increase methotrexate serum levels. Laboratory studies demonstrate that methotrexate may be displaced from plasma albumin by various compounds including sulfonamides, salicylates, tetracyclines, chloramphenicol and phenytoin. Drug-Drug Interactions Non-steroidal Anti-inflammatory Drugs (NSAIDs) NSAIDs should not be administered prior to or concomitantly with high doses of methotrexate. Concomitant administration of NSAIDs with high-dose methotrexate therapy has been reported to elevate and prolong serum methotrexate levels, resulting in deaths from severe hematologic (including bone marrow suppression and aplastic anemia) and gastrointestinal toxicity. These drugs have been reported to reduce the tubular secretion of methotrexate, in an animal model, and may enhance its toxicity by increasing methotrexate levels. Caution should be used when NSAIDs and salicylates are administered concomitantly with lower doses of methotrexate . In treating rheumatoid arthritis with methotrexate, the possibility of increased toxicity with concomitant use of NSAIDs including salicylates has not been fully explored. Despite the potential interactions, studies of methotrexate in patients with rheumatoid arthritis have usually included concurrent use of constant dosage regimens of NSAIDs without apparent problems. It should be appreciated however, that the doses used in rheumatoid arthritis (7.5 to 15 mg/week) are somewhat lower than those used in psoriasis and that larger doses could lead to toxicity. Disease Modifying Antirheumatic drugs (DMARDs) Combined use of methotrexate with gold, penicillamine, hydroxychloroquine, or sulfasalazine has not been studied and may increase the incidence of adverse effects. Amiodarone Amiodarone administration to patients receiving methotrexate treatment for psoriasis has induced ulcerated skin lesions. L-asparaginase The administration of L-asparaginase has been reported to antagonize the effect of methotrexate. Diuretics Bone marrow suppression and decreased folate levels have been described in the concomitant administration of triamterene and methotrexate. Leflunomide Methotrexate in combination with leflunomide may increase the risk of pancytopenia. Drugs Highly Bound to Plasma Proteins Methotrexate is partially bound to serum albumin, and toxicity may be increased because of displacement by other highly bound drugs, such as sulfonylureas, aminobenzoic acid, salicylates, phenylbutazone, phenytoin, sulfonamides, some antibiotics such as penicillins, tetracycline, pristinamycin, probenecid, and chloramphenicol. Packed Red Blood Cells Care should be exercised whenever packed red blood cells and methotrexate are given concurrently. Patients receiving 24-hr methotrexate infusion and subsequent transfusions have showed enhanced toxicity probably resulting from prolonged high serum-methotrexate concentrations. Probenecid Renal tubular transport is also diminished by probenecid; use of methotrexate with this drug should be carefully monitored. Proton Pump Inhibitors Use caution when administering high-dose methotrexate to patients receiving proton pump inhibitor (PPI) therapy. Concomitant use of PPIs and high-dose methotrexate should be avoided especially in patients with renal impairment. Case reports and published population pharmacokinetic studies suggest that concomitant use of some PPIs, such as omeprazole, esomeprazole, and pantoprazole, with methotrexate (primarily at high dose), may elevate and prolong serum levels of methotrexate and/or its metabolite hydromethotrexate, possibly leading to methotrexate toxicities. In two of these cases, delayed methotrexate elimination was observed when high-dose methotrexate was co-administered with PPIs, but was not observed when methotrexate was co-administered with ranitidine. However, no formal drug interaction studies of methotrexate with ranitidine have been conducted. Psoralen Plus Ultraviolet Light (PUVA) Therapy Skin cancer has been reported in patients with psoriasis or mycosis fungoides (a cutaneous T-cell lymphoma) receiving a concomitant treatment with methotrexate plus PUVA therapy. Nephrotoxic Drugs In the treatment of patients with osteosarcoma, caution must be exercised if high-dose methotrexate is administered in combination with a potentially nephrotoxic chemotherapeutic agent (e.g., cisplatin). Methotrexate clearance is decreased by cisplatinum. Although not documented, other nephrotoxic drugs such as aminoglycosides, Amphotericin B and Cyclosporin could theoretically increase methotrexate toxicity by decreasing its elimination. Nitrous Oxide The use of nitrous oxide anesthesia potentiates the effect of methotrexate on folate metabolism, yielding increased toxicity such as severe, unpredictable myelosuppression, stomatitis, neurotoxicity (with intrathecal administration of methotrexate) and nephritis (see CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS: Renal). In case of accidental co-administration, this effect can be reduced by the use of leucovorin rescue. Penicillins and Sulfonamides Penicillins and sulfonamides may reduce the renal clearance of methotrexate; hematologic and gastrointestinal toxicity have been observed in combination with methotrexate. Use of methotrexate with penicillins should be carefully monitored. Ciprofloxacin Renal tubular transport is diminished by ciprofloxacin; use of methotrexate with this drug should be carefully monitored. Oral Antibiotics Oral antibiotics such as tetracycline, chloramphenicol, and nonabsorbable broad spectrum antibiotics, may decrease intestinal absorption of methotrexate or interfere with the enterohepatic circulation by inhibiting bowel flora and suppressing metabolism of the drug by bacteria. For example: Neomycin, Polymyxin B, Nystatin and Vancomycin decrease methotrexate absorption, whereas Kanamycin increases methotrexate absorption. Trimethoprim/sulfamethoxazole has been reported rarely to increase bone marrow suppression in patients receiving methotrexate, probably by decreased tubular secretion and/or an additive antifolate effect. Concurrent use of the anti-protozoal pyrimethamine may increase the toxic effects of methotrexate because of an additive antifolate effect. Theophylline Methotrexate may decrease the clearance of theophylline; theophylline levels should be monitored when used concurrently with methotrexate. Mercaptopurine Methotrexate increases the plasma levels of mercaptopurine. Combination of methotrexate and mercaptopurine may therefore require dose adjustment. Vitamins Vitamin preparations containing folic acid or its derivatives may decrease responses to methotrexate. Preliminary animal and human studies have shown that small quantities of intravenously administered leucovorin enter the cerebrospinal fluid primarily as 5-methyl tetrahydrofolate and, in humans, remain 1 to 3 orders of magnitude lower than the usual methotrexate concentrations following intrathecal administration. However, high doses of leucovorin may reduce the efficacy of intrathecally administered methotrexate. In patients with rheumatoid arthritis or psoriasis, folic acid or folinic acid may reduce methotrexate toxicities such as gastrointestinal symptoms, stomatitis, alopecia, and elevated liver enzymes. Before taking a folate supplement, it is advisable to check B12 levels, particularly in adults over the age of 50, since folate administration can mask symptoms of B12 deficiency. Folate deficiency states may increase methotrexate toxicity. Radiotherapy Methotrexate given concomitantly with radiotherapy may increase the risk of soft tissue necrosis and osteonecrosis. Hepatoxins The potential for increased hepatotoxicity when methotrexate is administered with other hepatotoxic agents has not been evaluated. However, hepatotoxicity has been reported in such cases. Therefore, patients receiving concomitant therapy with methotrexate and other potential hepatotoxic agents (e.g., leflunomide, azathioprine, sulfasalazine, retinoids) should be closely monitored for possible increased risk of hepatotoxicity. Cytarabine and other cytotoxic agents Methotrexate given concomitantly with I.V. cytarabine may increase the risk of severe neurologic adverse events such as headache, paralysis, coma and stroke-like episodes (see WARNINGS AND PRECAUTIONS: Neurologic). Combined use of methotrexate with other cytotoxic agents has not been studied and may increase the incidence of adverse effects. Drug-Herb Interactions The effects of herbal products on the pharmacokinetics of methotrexate have not been studied. Drug-Laboratory Interactions Interactions with laboratory tests have not been established. Drug-Lifestyle Interactions Use of alcohol with methotrexate is contraindicated (see CONTRAINDICATIONS). The effects of smoking, on the pharmacokinetics of methotrexate have not been specifically studied. Some of the effects (e.g., dizziness and fatigue) may have an influence on the ability to drive or operate machinery. DOSAGE AND ADMINISTRATION WARNINGS The dose must be adjusted carefully depending on the body surface area if methotrexate is used for the treatment of tumour diseases. Fatal cases of intoxication have been reported after administration of incorrect calculated doses. Health care professionals and patients should be fully informed about toxic effects. Treatment should be initiated by or occur in consultation with a doctor with significant experience in cytostatic treatment. Abitrexate Teva may be administered by intramuscular, intravenous (bolus injection or infusion), intrathecal, intra- ventricular or intra-arterial route. For intrathecal administration, Abitrexate Teva is administered as a 1 mg/ml solution, using an appropriate sterile preservative-free medium such as Sodium Chloride Injection. Dosages are based on the patient's bodyweight or surface area, except in the case of intrathecal or intra-ventricular administration, when a maximum dose of 15 mg is recommended. Dosage should be reduced in cases of hematological deficiency and hepatic or renal impairment. When administered by infusion, Abitrexate Teva should only be diluted with normal saline. Larger doses (more than 100 mg) are usually administered by intravenous infusion over periods not exceeding 24 hours. Part of the dose may be administered as an initial rapid intravenous injection. Abitrexate Teva has been used with beneficial effects in a wide variety of neoplastic diseases, alone and in combination with other cytotoxic agents, hormones, immunotherapy, radiotherapy or surgery. Therefore, dosage schedules vary considerably depending on the clinical use, particularly when intermittent high-dose regimens are followed by the administration of calcium leucovorin in order to rescue normal cells from toxic effects. Dosage regimens for calcium leucovorin rescue are discussed at the end of this section. The following are some examples of the dosages of Abitrexate Teva that have been used for particular indications: Choriocarcinoma and Other Trophoblastic Tumors By the intramuscular route, in doses of 15-30 mg daily for a 5-day course. Such courses are usually repeated 3-5 times as required, with rest periods of 1 or more weeks between courses, until any toxic symptoms subside. The effectiveness of therapy is ordinarily evaluated by 24-hour quantitative analysis of urinary human chorionic gonadotrophin (HCG), which should return to normal or less than 50 IU/24 hours, usually after the 3rd or 4th course of treatment, and also usually followed by a complete resolution of measurable lesions in 4-6 weeks. After the normalization of HCG, 1 or 2 courses of Abitrexate Teva are usually recommended. Before each course of the drug, careful clinical assessment is essential. Higher doses of up to 60 mg i.m. every 48 hours may be administered for 4 doses, followed by calcium leucovorin rescue. This course is repeated at 7-day intervals until levels of urinary HCG return to normal. Not less than 4 courses of treatment are usually necessary. Patients with complications, such as extensive metastases, may be treated with Abitrexate Teva in cyclic combination with other cytotoxic drugs. Chorioadenoma Destruens and Hydatidiform Mole Since hydatidiform mole may be followed by choriocarcinoma, prophylactic chemotherapy with Abitrexate Teva has been recommended. Chorioadenoma destruens is considered to be an invasive form of hydatidiform mole. Abitrexate Teva is administered in these disease states in doses similar to those recommended for choriocarcinoma. Lymphoblastic Leukemia Daily administration of Abitrexate Teva 3.3 mg/m2, in combination with prednisone 60 mg/m2, is used as induction therapy in acute lymphatic (lymphoblastic) leukemia in children and young adolescents. Abitrexate Teva alone, or in combination with other agents, appears to be a drug of choice for securing maintenance of drug-induced remissions. When remission is achieved and supportive care has produced general clinical improvement, maintenance therapy is initiated with intramuscular methotrexate 30 mg/m2, twice weekly. It has also been administered intravenously in doses of 2.5 mg/kg body weight, every 14 days. If relapse does occur, reinduction of remission can again usually be obtained by repeating the initial induction regimen. Meningeal Leukemia Administer 12 mg/m2 intrathecally, or an empirical dose of 15 mg. Dilute methotrexate to a concentration of 1 mg/ml using a sterile, preservative-free medium such as 0.9% Sodium Chloride Injection. Administer at intervals of 2-5 days, and repeat until the CSF cell count returns to normal. Then administer one additional dose. Administration at intervals of less than 1 week may result in increased subacute toxicity. For prophylaxis against meningeal leukemia, the dosage is the same as for treatment, except for the intervals of administration. The CSF volume is dependent on age, and not on body surface area (BSA). The CSF is at 40% of the adult volume at birth and reaches the adult volume in several years. Intrathecal methotrexate 12 mg/m2 (maximum 15 mg) has resulted in low CSF methotrexate concentrations and reduced efficacy in children, and high concentrations and neurotoxicity in adults. The following dosage regimen is based on age instead of BSA, and appears to result in more consistent CSF methotrexate concentrations and less neurotoxicity. Intrathecal Methotrexate Dosage According to Age. Age (years) Dose (mg) Under 1 6 1 8 2 10 Over 3* 12 *equal or higher than 3 years of age. Because the CSF volume and turnover may decrease with age, a dose reduction may be indicated in elderly patients. Lymphomas In stage III, give methotrexate concomitantly with other antitumor agents. Treatment in all stages generally consists of several courses with 7-10 day rest periods between each course. Lymphosarcomas in stage III may respond to combined drug therapy with methotrexate 0.625-2.5 mg/kg body weight/day. Mycosis Fungoides Although the usual treatment is by orally-administered methotrexate, methotrexate has also been given intramuscularly in doses of 50 mg once a week, or 25 mg twice weekly. Breast Cancer Abitrexate Teva in intravenous doses of 10-60 mg/m2 is commonly included in cyclical combination regimens with other cytotoxic drugs in the treatment of advanced breast cancer. Similar regimens have also been used as adjuvant therapy in early cases following mastectomy and/or radiotherapy. Osteosarcoma Effective therapy requires several cytotoxic chemotherapeutic agents. In addition to high-dose methotrexate with leucovorin rescue, these agents may include doxorubicin, cisplatin and the combination of bleomycin, cyclophosphamide and dactinomycin (BCD) in the doses and schedule shown in the Table below. The starting dose for high-dose methotrexate treatment is 12g/m2. If this is insufficient to produce a peak serum concentration of 1,000 micromole per L (0.001 mol/l) at the end of the methotrexate infusion, the dose may be increased to 15g/m2 in subsequent treatments. If the patient is vomiting or unable to tolerate leucovorin orally, administer leucovorin I.V. or I.M. at the same dose and schedule. Chemotherapy Regimens for Osteosarcoma DRUG* DOSE* TREATMENT WEEK AFTER SURGERY Methotrexate 12 g/m2 I.V. as 4-hour 4,5,6,7,11,12,15, infusion (starting dose) 16,29,30,44,45 Leucovorin 15 mg orally every 6 hours for 10 doses, starting 24 hours after start of methotrexate infusion Doxorubicin as a single 30mg/m2/day I.V. x 3 days 8,17 drug** Doxorubicin** 50 mg/m2 I.V. 20,23,33,36 Cisplatin** 100 mg/m2 I.V. 20,23,33,36 Bleomycin** 15 units/m2 I.V. x 2 days 2,13,26,39,42 Cyclophosphamide** 600 mg/m2 I.V. x 2 days 2,13,26,39,42 Dactinomycin** 0.6 mg/m2 I.V. x 2 days 2,13,26,39,42 * Link MP, Goorin AM, Miseer AW et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity, N. Engl. J. Med. 1986;3 14(25):1600-6. ** See each respective monograph for more complete information. Dosage modifications may be necessary because of drug-induced toxicity. Bronchogenic Carcinoma Intravenous infusions of 20-100 mg/m2 of Abitrexate Teva have been included in cyclical combination regimens for the treatment of advanced tumors. Higher doses of Abitrexate Teva with calcium leucovorin rescue may also be employed as sole treatment. Head and Neck Cancer Intravenous infusions of 240-1,080 mg/m2 of Abitrexate Teva with calcium leucovorin rescue may be used both as preoperative adjuvant therapy and in the treatment of advanced tumors. Intra-arterial infusions of Abitrexate Teva are indicated for certain head and neck cancers, although this route of administration is not used extensively. Bladder Carcinoma Intravenous injections or infusions of Abitrexate Teva in doses up to 100 mg every 1-2 weeks may be used in the treatment of bladder carcinoma. Diuretics and hydration are employed in an attempt to reduce the excessive drug toxicity that may occur in patients with renal impairment. Psoriasis Patients should be fully informed of the potential risks involved, and should be under the constant supervision of the treating physician. The usual dose in cases of severe, uncontrolled psoriasis unresponsive to conventional therapy is 10-25 mg, administered intramuscularly or intravenously once a week and adjusted according to the patient's response. Rheumatoid Arthritis Note: The following recommendation is based on clinical studies whose tabulated dosages, as well as the references appear at the end of the paragraph. Initially, 10 mg/week may be administered either intramuscularly or intravenously. The dosage may be increased to 25 mg/week. Duration of treatment varied in clinical studies from 6 weeks to 13 weeks. An intramuscular dosage of 15 mg/week has been administered over a period of 6 months. An initial dosage of 10 mg/week IV, increased to a maximum of 50 mg/week I.V. has been administered over a period of 2 months. Tabulated Summary of Dosages for the Use of Methotrexate (Parenteral) in Rheumatoid Arthritis STUDY DOSE Herman (1) 10 mg/m2, I.V. Ahern (2) 15 mg , oral 15 mg, I.V. Campbell (3) 30 mg/m2, oral 30 mg/m2, I.V. 30 mg/m2,, I.M. Steinson (4) 7.5 mg-25 mg per week; I.M./oral Michaels (5) 10 mg-50 mg per week, I.V. Andersen (6) 10 mg per week, I.M., increased up to 25 mg if required. Thompson (7) 10 mg or 25 mg per week, I.M. Hoffmeister (8) 10 mg-15 mg per week, oral and I.M. Weinstein (9) 7.5 mg-25 mg per week, oral and I.M. Szanto (10) 5 mg-15 mg per week oral/I.M. Tishler (11) 12.5 mg (7.5 mg-15 mg) per week, oral/I.M. Suarez-Almazor (12) 10 mg per week I.M. Rau (13) 15 mg per week I.M. References 1. Herman, R.A., Veng-Pedersen, P., Hoffman, J., Koehnke, R., and Furst, D.E.: Pharmacokinetics of Low-Dose Methotrexate in Rheumatoid Arthritis Patients. J. Pharm. Sci. 78: 165-171, 1989. 2. Ahern, M., Booth, J., Loxton, A., McCarthy, P.M., Meffin, P., Kevant, S.: Methotrexate Kinetics in Rheumatoid Arthritis: Is there an Interaction with Nonsteroidal Anti-Inflammatory Drugs. J. Rheumatol. 15: 1356-1360, 1988. 3. Campbell, M.A., Perrier, D.G., Dorr, R. T., et. al.: Methotrexate Bioavailability .Cancer Treat. Rev. 69: 833-838, 1985. 4. Steinson, K., Weinstein, A., Korn, J., Abeles, M.: Low-Dose Methotrexate in Rheumatoid Arthritis. J. Rheumatol. 9: 860-866, 1982. 5. Michaels, R. M., Nashel, D.J., Leonard, A., Sliwinski, A.J., Derbes, A. J.: Weekly Intravenous Methotrexate in the Treatment of Rheumatoid Arthritis. Arthritis Rheum. 25: 339-341, 1982. 6. Andersen, P.A., West S.G., O'Dell, J. R., Via C.S.,Claypool, R. G., Kotzin B. L. Weekly Pulse Methotrexate in Rheumatoid Arthritis. Clinical and Immunologic Effects in a Randomized, Double-Blind Study. Ann. Intern. Med., 103: 489-496, 1985. 7. Thompson, R. N., Watts, C., Edelman, J., Russell, A. S.,: A Controlled Two-Center Trial of Parenteral Methotrexate Therapy for Refractory Rheumatoid Arthritis. J. Rheumatol. 11: 760-763, 1984. 8. Hoffmeister, R. T., :Methotrexate in Rheumatoid Arthritis: 15 Years Experience. Am. J. Med. 75: 69-73, 1983. 9. Weinstein, A., Marlowe, S., Korn, J., Farouhan, F. Low-Dose Methotrexate Treatment of Rheumatoid Arthritis. Long-Term Observations. Am. J. Med. 79: 331-337, 1985. 10. Szanto, E., Low-Dose Methotrexate in Rheumatoid Arthritis: Effect and Tolerance. An Open Trial and a Double- Blind Randomized Study. Scand. J. Rheumatol. 15: 97-102, 1986. 11. Tishler, M., Caspi, D., Rosenbach, T. O., Fishel, B., Wigler, I., Segal, R., Gazit, E., Yaron, M.: Methotrexate in Rheumatoid Arthritis; A Prospective Study in Israeli Patients with immunogenetic Correlations. Ann. Rheum. Dis. 47: 654-659, 1988. 12. Suarz-Almazor M. E., Fitzgerald, A., Grace, M., Russell, A. S.: a Randomized Controlled Trial of Parenteral Methotrexate Compared with Sodium Aurothiomalate (Myochrysine) in the Treatment of Rheumatoid Arthritis. J. Rheumatol. 15: 753-756, 1988. 13. Rau, R., Herborn, G., Kargen, T., Menninger, H., Elhardt, D.A Blinded Randomized Trial of Methotrexate and Gold Sodium Aurothiomalate in Early Erosive Rheumatoid Arthritis. Arthritis Rheum. 32: S43, 1981 (Abstract). Particular attention should be given to the appearance of liver toxicity by performing liver function tests before initiating Abitrexate Teva treatment, and repeating the tests at 2-4 month intervals during therapy. Therapy should not be instituted, or should be discontinued, if any abnormality of liver function tests or of a liver biopsy is present or develops during therapy. Such abnormalities should return to normal within 2 weeks, after which, treatment may be recommended at the discretion of the physician. The use of Abitrexate Teva may permit the return to conventional topical therapy which should be encouraged. Calcium Leucovorin Rescue When administering high doses of methotrexate, the following guidelines for methotrexate therapy with leucovorin rescue should be closely observed: • Methotrexate administration should be delayed until recovery if: the WBC count is less than 1500mm3 the neutrophil count is less than 500mm3 the platelet count is less than 75,000mm3 the serum bilirubin level is more than 1.2 mg/dl the ALT level is more than 450 U mucositis is present, until there is evidence of healing persistent pleural effusion and ascite are present; drain dry prior to infusion • Adequate renal function must be documented. • Serum creatinine must be normal and creatinine clearance must be more than 60 ml/min. before the initiating of therapy. • Serum creatinine must be measured prior to each subsequent course of therapy. If serum creatinine has increased by 50% or more in comparison to a prior value, the creatinine clearance must be measured and documented to be more than 60 ml/min. (even if the serum creatinine is still within the normal range). • Patients must be well hydrated, and must be treated with sodium bicarbonate for urinary alkalinization. Administer 1,000 ml/m2 of intravenous fluid over 6 hours prior to initiating of the methotrexate infusion. Continue hydration at 125 ml/m2/hour (3L/m2/day) during methotrexate infusion, and for 2 days after the infusion has been completed. • Alkalinize urine to maintain a pH above 7.0 during methotrexate infusion and leucovorin calcium therapy by giving sodium bicarbonate orally or by incorporation into a separate I.V. solution. • Repeat serum creatinine and serum methotrexate 24 hours after starting methotrexate, and at least once daily until the methotrexate level is below 5x10-8 mol/l (0.05 micromolar). Leucovorin Rescue Schedules Following Treatment with Higher Doses of Methotrexate CLINICAL SITUATION LABORATORY FINDINGS LEUCOVORIN CALCIUM DOSAGE AND DURATION Normal Methotrexate Serum methotrexate level approximately 10 15 mg PO or I.V. every 6 Elimination micromolar at 24 hours after administration, 1 hours for 60 hours (10 doses micromolar at 48 hours and less than 0.2 starting at 24 hours after start micromolar at 72 hours of methotrexate infusion). Delayed Late Serum methotrexate level remaining above 0.2 Continue 15 mg PO or I.V. Methotrexate Elimination micromolar at 72 hours and more than 0.05 every 6 hours until micromolar at 96 hours after administration. methotrexate level is less than 0.05 micromolar. Delayed Early Serum methotrexate level of equal or higher than 150 mg I.V. every 3 hours, Methotrexate Elimination 50 micromolar at 24 hours or equal or higher than until methotrexate level is less and/or Evidence of Acute 5 micromolar at 48 hours after administration, or a than 1 micromolar; then 15 mg Renal Injury. 100% or greater increase in serum creatinine level I.V. every 3 hours, until at 24 hours after methotrexate administration (e.g. methotrexate level is less than an increase from 0.5 mg/dl to a level equal or 0.05 micromolar. higher than 1.0 mg/dl or more) Patients who experience delayed/early methotrexate elimination are likely to develop irreversible oliguric renal failure. In addition to appropriate leucovorin therapy, these patients require continuing hydration and urinary alkalinization and close monitoring of fluid and electrolyte status, until the serum methotrexate level has fallen to less than 0.05 micromolar and the renal failure has resolved. Some patients will have abnormalities in methotrexate elimination or abnormalities in renal function following methotrexate administration which are significant, but less severe, than those described in the Table. These abnormalities may, or may not be, associated with significant clinical toxicity. If significant clinical toxicity is observed, leucovorin rescue should be extended for an additional 24 hours (total of 14 doses over 84 hours) in subsequent courses of therapy. When laboratory abnormalities or clinical toxicities are observed, the possibility that the patient is taking other medications which interact with methotrexate should be considered. Older People Dose reduction should be considered in elderly patient due to reduced liver and kidney function as well as reserves which occur with increased age. Hepatic Function Impairment If the bilirubin is between 3-5, or AST more than 180, dosage should be reduced by 25%. If bilirubin is more than 5, omit the dose. Methotrexate should be administered with great caution, if at all, to patients with significant current or previous liver disease, especially when caused by alcohol. Methotrexate is contraindicated if bilirubin values are >5 mg/dl (85.5 μmol/L). Missed Dose If a scheduled dose is missed, contact your doctor for instructions. Administration Do not use this medicine if the solution is not clear. Abitrexate Teva solution can be further diluted with 0.9% Sodium Chloride Injection or 5% Dextrose Injection. Abitrexate Teva diluted solution stored between 15-250C and protected from light has a chemical and physical in-use stability of 24 hours. From a microbiological point of view, unless the method of dilution precludes the risk of microbial contamination, the product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user. Since methotrexate is poorly soluble in acid media, use of potassium chloride solution is not advisable. If a preservative-free diluent is used, the solution should be used immediately because of the possibility of microbial growth. Due to the number of brands available, stability data of methotrexate in plastic syringes and bags are not available. Unused preservative-free products should be discarded due to the possibility of microbial growth. Incompatibilities: Other drugs should not be mixed with methotrexate in the same infusion bottle. Methotrexate has been reported to be incompatible with cytarabine, fluorouracil, and prednisolone sodium phosphate; however, its incompatibility with fluorouracil has been questioned and subsequent studies documented in the literature indicate that methotrexate and cytarabine are physically and chemically stable in intravenous admixtures over a range of concentrations and in a variety of typical vehicles. A mixture of methotrexate with cytarabine and hydrocortisone sodium succinate in various infusion fluids has been reported to be visually compatible for at least 8 hours at 25°C, although precipitation did not occur on storage for several days. In general, compatibility of any medicinal product admixed with Abitrexate Teva must be assured prior to patient administration. Contact with acidic solutions should be avoided since Abitrexate Teva is sparingly soluble in acid media and precipitation may occur (see WARNINGS AND PRECAUTIONS for clinical incompatibilities).

שימוש לפי פנקס קופ''ח כללית 1994
Leukemias, non-hodgkin's lymphomas, breast, head and lung carcinoma, choriocarcinoma, osteogenic sarcoma. Severe psoriasis, rheumatoid arthritis unresponsive to conventional therapy, mycosis fungoides
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
