Quest for the right Drug
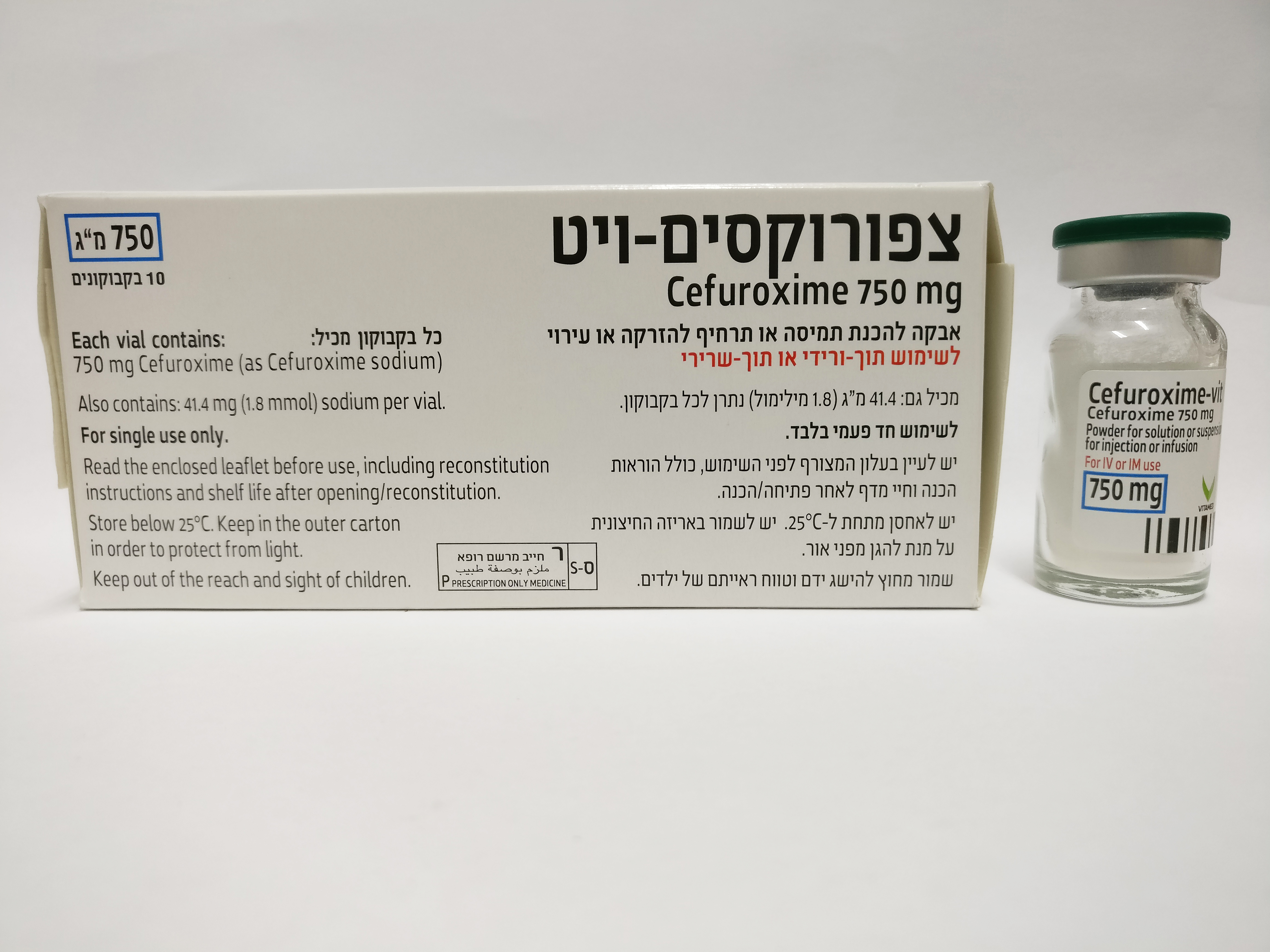
צפורוקסים - ויט CEFUROXIME - VIT (CEFUROXIME AS SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי : I.M, I.V
צורת מינון:
אין פרטים : POWDER FOR SOLUTION OR SUSPENSION FOR INJECTION OR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The most common adverse reactions are neutropenia, eosinophilia, transient rise in liver enzymes or bilirubin, particularly in patients with pre-existing liver disease, but there is no evidence of harm to the liver and injection site reactions. The frequency categories assigned to the adverse reactions below are estimates, as for most reactions suitable data for calculating incidence are not available. In addition the incidence of adverse reactions associated with cefuroxime sodium may vary according to the indication. Data from clinical trials were used to determine the frequency of very common to rare adverse reactions. The frequencies assigned to all other adverse reactions (i.e. those occurring at <1/10,000) were mainly determined using post-marketing data, and refer to a reporting rate rather than a true frequency. Treatment related adverse reactions, all grades, are listed below by MedDRA body system organ class, frequency and grade of severity. The following convention has been utilised for the classification of frequency: very common ≥1/10; common ≥ 1/100 to < 1/10; uncommon ≥ 1/1,000 to < 1/100; rare ≥ 1/10,000 to < 1/1,000; very rare < 1/10,000 and not known (cannot be estimated from the available data). System organ Common Uncommon Not known class Infections and Candida infestations overgrowth, overgrowth of Clostridioides difficile Blood and neutropenia, eosinophilia, leukopenia, positive thrombocytopenia, lymphatic decreased haemoglobin Coomb’s test haemolytic system concentration anaemia disorders Immune drug fever, system interstitial disorders nephritis, anaphylaxis, cutaneous vasculitis Gastrointestinal gastrointestinal pseudomembranous disorders disturbance colitis (see section 4.4) Hepatobiliary transient rise in liver transient rise in disorders enzymes bilirubin Skin and skin rash, urticaria and erythema subcutaneous pruritus multiforme, toxic tissue disorders epidermal necrolysis and Stevens-Johnson syndrome, angioneurotic oedema Renal and elevations in serum urinary creatinine, disorders elevations in blood urea nitrogen and decreased creatinine clearance (see section 4.4) General injection site reactions disorders and which may include pain administration and thrombophlebitis site conditions Description of selected adverse reactions Cephalosporins as a class tend to be absorbed onto the surface of red cell membranes and react with antibodies directed against the drug to produce a positive Coomb’s test (which can interfere with cross matching of blood) and very rarely haemolytic anaemia. Transient rises in serum liver enzymes or bilirubin have been observed which are usually reversible. Pain at the intramuscular injection site is more likely at higher doses. However, it is unlikely to be a cause for discontinuation of treatment. Paediatric population The safety profile for cefuroxime sodium in children is consistent with the profile in adults. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
