Quest for the right Drug
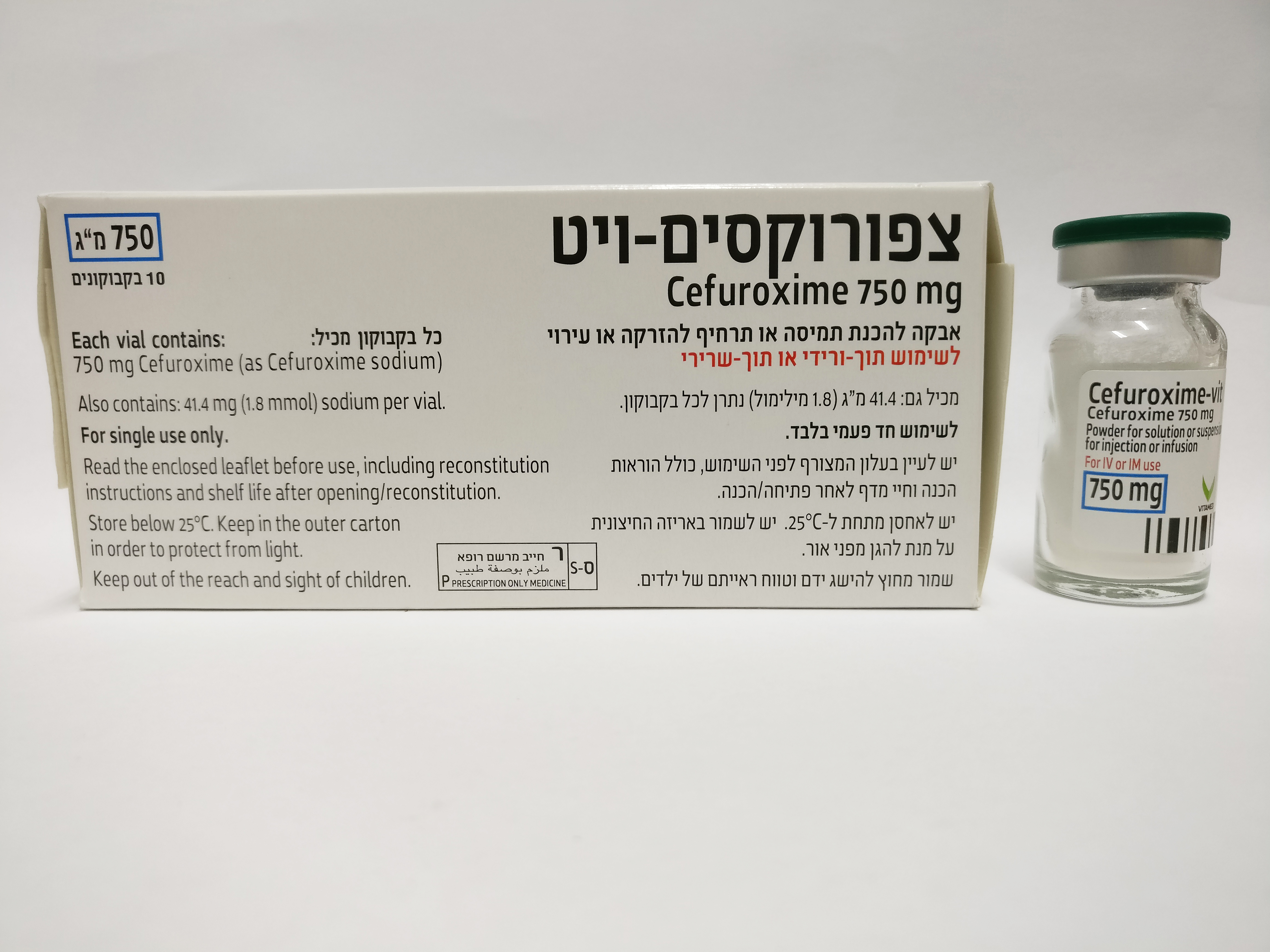
צפורוקסים - ויט CEFUROXIME - VIT (CEFUROXIME AS SODIUM)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי : I.M, I.V
צורת מינון:
אין פרטים : POWDER FOR SOLUTION OR SUSPENSION FOR INJECTION OR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Hypersensitivity reactions As with all beta-lactam antibacterial agents, serious and occasionally fatal hypersensitivity reactions have been reported. In case of severe hypersensitivity reactions, treatment with cefuroxime must be discontinued immediately and adequate emergency measures must be initiated. Before beginning treatment, it should be established whether the patient has a history of severe hypersensitivity reactions to cefuroxime, to other cephalosporins or to any other type of beta-lactam agent. Caution should be used if cefuroxime is given to patients with a history of non-severe hypersensitivity to other beta-lactam agents. Concurrent treatment with potent diuretics or aminoglycosides Cephalosporin antibiotics at high dosage should be given with caution to patients receiving concurrent treatment with potent diuretics such as furosemide or aminoglycosides. Renal impairment has been reported during use of these combinations. Renal function should be monitored in the elderly and those with known pre-existing renal impairment (see section 4.2). Overgrowth of non-susceptible microorganisms Use of cefuroxime may result in the overgrowth of Candida. Prolonged use may also result in the overgrowth of other non-susceptible microorganisms (e.g. enterococci and Clostridioides difficile), which may require interruption of treatment (see section 4.8). Antibacterial agent–associated pseudomembranous colitis has been reported with use of cefuroxime and may range in severity from mild to life threatening. This diagnosis should be considered in patients with diarrhoea during or subsequent to the administration of cefuroxime (see section 4.8). Discontinuation of therapy with cefuroxime and the administration of specific treatment for Clostridioides difficile should be considered. Medicinal products that inhibit peristalsis should not be given. Intracameral use and eye disorders Cefuroxime - Vit is not formulated for intracameral use. Individual cases and clusters of serious ocular adverse reactions have been reported following unapproved intracameral use of cefuroxime sodium compounded from vials approved for intravenous/intramuscular administration. These reactions included macular oedema, retinal oedema, retinal detachment, retinal toxicity, visual impairment, visual acuity reduced, vision blurred, corneal opacity and corneal oedema. Intra-abdominal infections Due to its spectrum of activity, cefuroxime is not suitable for the treatment of infections caused by Gram-negative non-fermenting bacteria or by Bacteroides fragilis (see section 5.1). Interference with diagnostic tests The development of a positive Coomb’s Test associated with the use of cefuroxime may interfere with cross matching of blood (see section 4.8). Slight interference with copper reduction methods (Benedict's, Fehling's, Clinitest) may be observed. However, this should not lead to false-positive results, as may be experienced with some other cephalosporins. As a false negative result may occur in the ferricyanide test, it is recommended that either the glucose oxidase or hexokinase methods are used to determine blood/plasma glucose levels in patients receiving cefuroxime sodium. Important information about excipients 750 mg vial: This medicinal product contains approximately 42 mg (1.8 mmol) sodium per vial, equivalent to 2.1% of the WHO recommended maximum daily intake of 2 g sodium for an adult.
Effects on Driving
4.7 Effects on ability to drive and use machines No studies on the effects of cefuroxime on the ability to drive and use machines have been performed. However, based on known adverse reactions, cefuroxime is unlikely to have an effect on the ability to drive and use machines.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
