Quest for the right Drug
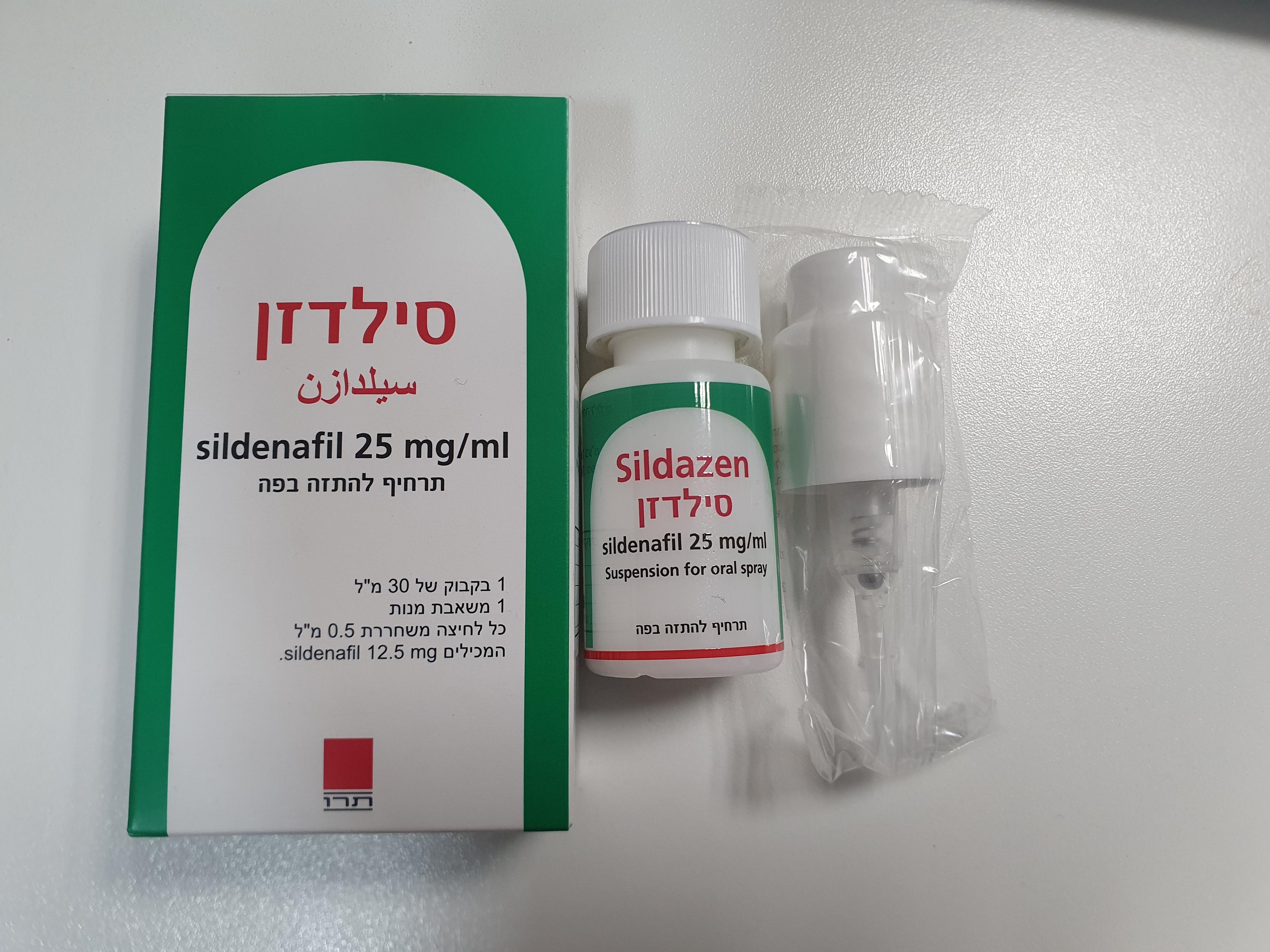
סילדזן SILDAZEN (SILDENAFIL AS CITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : ORAL
צורת מינון:
אין פרטים : SUSPENSION FOR ORAL SPRAY
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Interactions : אינטראקציות
4.5 Interaction with other medicinal products and other forms of interaction Effects of other medicinal products on sildenafil In vitro studies Sildenafil metabolism is mediated primarily by the cytochrome P450 (CYP) isoforms 3A4 (mostly) and 2C9 (to a lesser extent). Therefore, inhibitors of these isoenzymes may reduce sildenafil clearance and inducers of these isoenzymes may increase sildenafil clearance. In vivo studies Pharmacokinetic analysis of clinical trial data indicated a reduction in sildenafil clearance when co-administered with CYP3A4 inhibitors (such as ketoconazole, erythromycin, cimetidine). Although no increased incidence of adverse events was observed in these patients, when sildenafil is administered concomitantly with CYP3A4 inhibitors, a starting dose of 25 mg (two actuations) should be considered. Co-administration of the HIV protease inhibitor ritonavir, which is a potent inhibitor of cytochrome P450, at steady state (500 mg twice daily) with sildenafil (100 mg single dose) resulted in a 300% (4-fold) increase in sildenafil Cmax and a 1,000% (11-fold) increase in sildenafil plasma AUC. At 24 hours, the plasma levels of sildenafil were still approximately 200 ng/mL, compared to approximately 5 ng/mL when sildenafil was administered alone. This is consistent with ritonavir’s marked effects on a large number of cytochrome P450 substrates. Sildenafil had no effect on the pharmacokinetics of ritonavir. Based on these pharmacokinetic results, co-administration of sildenafil with ritonavir is not advised (see section 4.4) and the maximum dose of sildenafil should under no circumstances exceed 25 mg (two actuations) in 48 hours. Co-administration of the HIV protease inhibitor saquinavir, also a cytochrome CYP3A4 inhibitor, at steady state (1,200 mg three times a day), with sildenafil (100 mg single dose) resulted in a 140% increase in sildenafil Cmax and a 210% increase in sildenafil AUC. Sildenafil had no effect Page 8 of 17 on the pharmacokinetics of saquinavir (see section 4.2). Stronger cytochrome CYP3A4 inhibitors such as ketoconazole and itraconazole would be expected to have greater effects. When a single 100 mg dose of sildenafil was administered with erythromycin, a moderate CYP3A4 inhibitor, there was a 182% increase in sildenafil systemic exposure (AUC) when a stable plasma concentration of erythromycin (500 mg twice daily for 5 days) was reached. In healthy male volunteers, there was no evidence of an effect of azithromycin (500 mg daily for 3 days) on the AUC, Cmax, tmax, elimination rate constant or subsequent half-life of sildenafil or its principal circulating metabolite. Cimetidine (800 mg), a cytochrome P450 inhibitor and non-specific CYP3A4 inhibitor, caused a 56% increase in plasma sildenafil concentrations when co-administered with sildenafil (50 mg) to healthy volunteers. Grapefruit juice is a weak inhibitor of cytochrome CYP3A4 gut wall metabolism and may give rise to modest increases in plasma levels of sildenafil. Single doses of antacid (magnesium hydroxide/aluminium hydroxide) did not affect the bioavailability of sildenafil. Although specific interaction studies have not been conducted for all medicinal products, pharmacokinetic data analysis showed no effect on the pharmacokinetics of sildenafil when co-administered with CYP2C9 inhibitors (such as tolbutamide, warfarin, phenytoin), CYP2D6 inhibitors (such as selective serotonin reuptake inhibitors, tricyclic antidepressants), thiazides and related diuretics, (loop and potassium sparing diuretics), angiotensin converting enzyme inhibitors, calcium channel blockers, beta-adrenoreceptor antagonists or inducers of CYP450 metabolism (such as rifampicin, barbiturates). In a study with healthy male volunteers, co-administration of the endothelin antagonist, bosentan (an inducer of CYP3A4 [moderate], of CYP2C9 and possibly of CYP2C19), at steady state (125 mg twice daily) with sildenafil at steady state (80 mg three times daily) resulted in a 62.6% and 55.4% decrease in sildenafil AUC and Cmax, respectively. Therefore, concomitant administration of strong CYP3A4 inducers, such as rifampin, is expected to cause greater decreases in plasma concentrations of sildenafil. Nicorandil is a hybrid of a potassium channel activator and nitrate. Due to its nitrate component, it has the potential to result in a serious interaction with sildenafil. Effects of sildenafil on other medicinal products In vitro studies Sildenafil is a weak inhibitor of the cytochrome P450 isoforms 1A2, 2C9, 2C19, 2D6, 2E1 and 3A4 (IC50 >150 μM). Given that sildenafil peak plasma concentrations, after the recommended doses, are approximately 1 μM, it is unlikely that sildenafil will alter the clearance of substrates of these isoenzymes. There are no data on the interaction of sildenafil and non-specific phosphodiesterase inhibitors such as theophylline or dipyridamole. Page 9 of 17 In vivo studies Consistent with its known effects on the nitric oxide/cGMP pathway (see section 5.1), sildenafil was shown to potentiate the hypotensive effects of nitrates. Its co-administration with nitric oxide donors or nitrates is therefore contraindicated (see section 4.3). Concomitant administration of sildenafil to patients taking alpha-blocker therapy may lead to symptomatic hypotension in a small number of more susceptible patients. This is most likely to occur within 4 hours of taking sildenafil (see sections 4.2 and 4.4). In three specific drug-drug interaction studies, the alpha-blocker doxazosin (4 mg and 8 mg) and sildenafil (25 mg, 50 mg or 100 mg) were administered simultaneously to patients with benign prostatic hyperplasia (BPH) who were already stabilised on doxazosin therapy. In these study populations, mean additional reductions in supine blood pressure of 7/7 mmHg, 9/5 mmHg and 8/4 mmHg, and mean additional reductions in standing blood pressure of 6/6 mmHg, 11/4 mmHg and 4/5 mmHg, respectively, were observed. When sildenafil and doxazosin were administered simultaneously to patients stabilised on doxazosin therapy, there were infrequent reports of patients who experienced symptomatic postural hypotension. These cases included dizziness or light-headedness, but not syncope. No significant interactions were observed when sildenafil (50 mg) was co-administered with tolbutamide (250 mg) or warfarin (40 mg), both of which are metabolised by CYP2C9. Sildenafil (50 mg) did not potentiate the increase in bleeding time caused by acetylsalicylic acid (150 mg). Sildenafil (50 mg) did not potentiate the hypotensive effects of alcohol in healthy volunteers with mean maximum blood alcohol levels of 80 mg/dL. The pooled analysis of all the data obtained on the following classes of antihypertensive medication: diuretics, beta-blockers, ACE inhibitors, angiotensin II antagonists, antihypertensive medicinal products (vasodilator and centrally-acting), adrenergic neurone blockers, calcium channel blockers and alpha-adrenoceptor blockers, showed no difference in the side effect profile in patients taking sildenafil compared to placebo treatment. In a specific interaction study, when sildenafil (100 mg) was co-administered with amlodipine in hypertensive patients, the mean additional reduction in supine systolic blood pressure was 8 mmHg. The corresponding additional reduction in supine diastolic blood pressure was 7 mmHg. These additional blood pressure reductions were of a similar magnitude to those observed when sildenafil was administered alone to healthy volunteers (see section 5.1). Sildenafil (100 mg) did not affect the steady state pharmacokinetics of the HIV protease inhibitors, saquinavir and ritonavir, both of which are cytochrome CYP3A4 substrates. In healthy male volunteers, administration of sildenafil at steady state (80 mg three times a day) resulted in a 49.8% increase in bosentan AUC and a 42% increase in bosentan Cmax (125 mg twice daily). The addition of a single dose of sildenafil to sacubitril/valsartan at steady state in patients with hypertension was associated with a significantly greater reduction in blood pressure compared to the administration of sacubitril/valsartan alone. Therefore, caution should be exercised when initiating sildenafil treatment in patients taking sacubitril/valsartan.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
