Quest for the right Drug
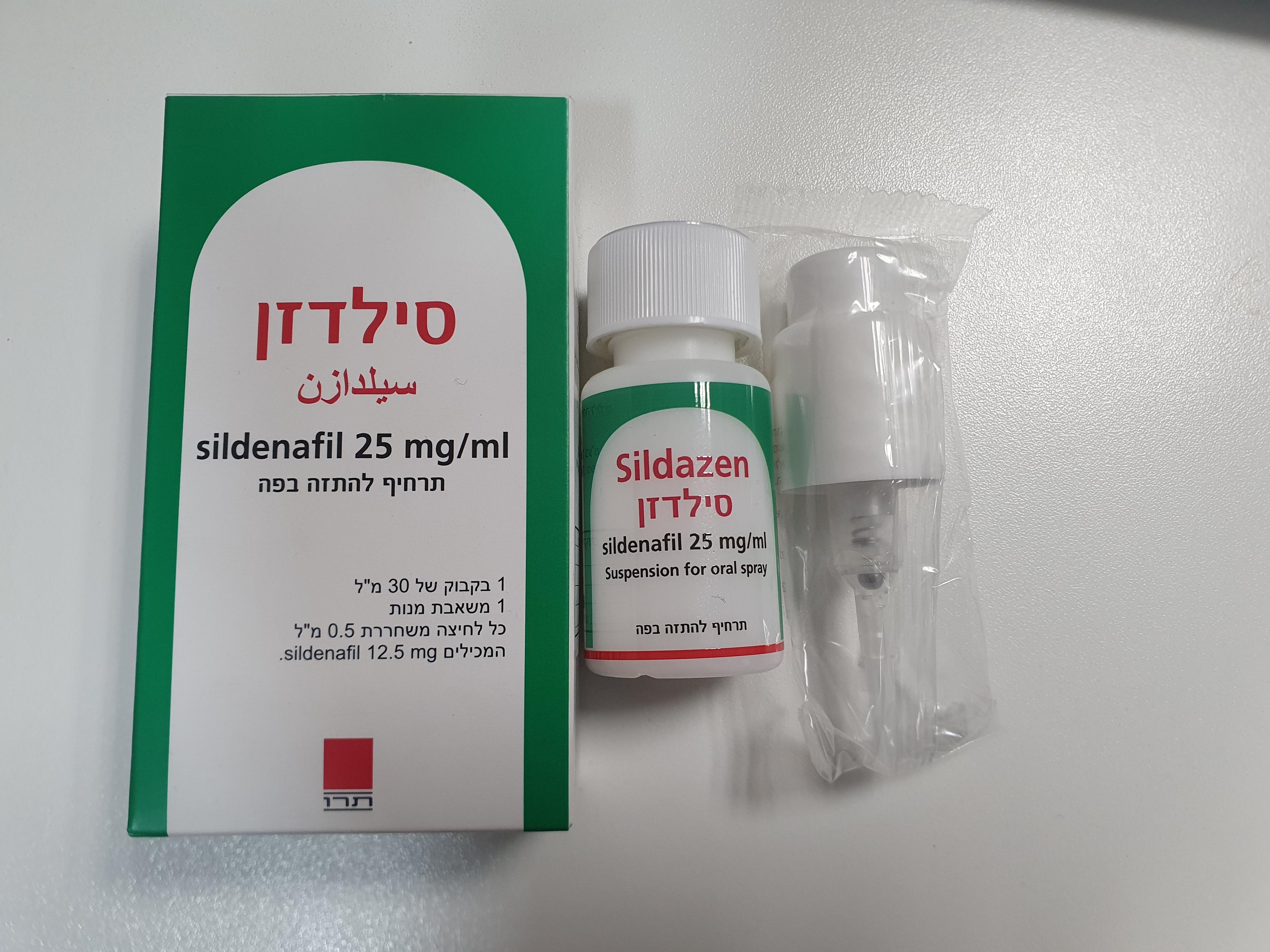
סילדזן SILDAZEN (SILDENAFIL AS CITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : ORAL
צורת מינון:
אין פרטים : SUSPENSION FOR ORAL SPRAY
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use A medical history and physical examination of the patient are advisable to diagnose erectile dysfunction and to determine potential underlying causes before considering pharmacological treatment. For oral administration only. Not to be used by other routes of administration. Cardiovascular risk factors Prior to initiating any treatment for erectile dysfunction, the physician should consider the cardiovascular status of their patients, since there is a degree of cardiac risk associated with sexual activity. Sildenafil has vasodilator properties, resulting in mild and transient decreases in blood pressure (see section 5.1). Prior to prescribing sildenafil, the physician should carefully consider whether their patients with certain underlying conditions could be adversely affected by such vasodilator effects, especially in combination with sexual activity. Patients with increased susceptibility to vasodilators include those with left ventricular outflow obstruction (e.g. aortic stenosis, hypertrophic obstructive cardiomyopathy) or those with the rare syndrome of multiple system atrophy manifesting as severely impaired autonomic control of blood pressure. Sildenafil potentiates the hypotensive effect of nitrates (see section 4.3). Serious cardiovascular events, including myocardial infarction, unstable angina, sudden cardiac death, ventricular arrhythmia, cerebrovascular haemorrhage, transient ischaemic attack, hypertension and hypotension have been reported post-marketing in temporal association with the use of sildenafil. Most, but not all, of these patients had pre-existing cardiovascular risk factors. Many events were reported to occur during or shortly after sexual intercourse and a few were reported to occur shortly after the use of sildenafil without sexual activity. It is not possible to determine whether these events are related directly to these factors or to other factors. Priapism Medicinal products for the treatment of erectile dysfunction, including sildenafil, should be used with caution in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis or Peyronie’s disease), or in patients with conditions which may predispose them to priapism (such as sickle cell anaemia, multiple myeloma or leukaemia). Prolonged erections and priapism have been reported with sildenafil in post-marketing experience. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency could result. Concomitant use with other PDE5 inhibitors or other treatments for erectile dysfunction The safety and efficacy of combinations of sildenafil with other PDE5 inhibitors, other pulmonary arterial hypertension (PAH) treatments containing sildenafil (REVATIO), or other treatments for erectile dysfunction have not been studied. Therefore, the use of such combinations is not recommended. Effects on vision Cases of visual defects have been reported spontaneously in connection with the intake of sildenafil and other PDE5 inhibitors (see section 4.8). Cases of non-arteritic anterior ischaemic optic neuropathy, a rare condition, have been reported spontaneously and in an observational study in connection with the intake of sildenafil and other PDE5 inhibitors (see section 4.8). Patients should be advised that, in the event of any sudden visual defect, they should stop taking sildenafil and consult a physician immediately (see section 4.3). Page 7 of 17 Concomitant use with ritonavir Co-administration of sildenafil with ritonavir is not advised (see section 4.5). Concomitant use with alpha-blockers Caution is advised when sildenafil is administered to patients taking an alpha-blocker, as the co-administration of both drugs may lead to symptomatic hypotension in a small number of more susceptible patients (see section 4.5). This is most likely to occur within 4 hours after taking sildenafil. In order to minimise the potential for developing postural hypotension, patients receiving alpha-blocker treatment should be haemodynamically stable on alpha-blocker therapy prior to initiating sildenafil treatment. Furthermore, initiation of sildenafil treatment at a dose of 25 mg (two actuations) should be considered (see section 4.2). In addition, physicians should advise their patients what to do in the event of postural hypotensive symptoms. Effects on bleeding Studies with human platelets indicate that sildenafil potentiates the antiaggregatory effect of sodium nitroprusside in vitro. There are no safety data on the administration of sildenafil to patients with bleeding disorders or active peptic ulcer. Therefore, sildenafil should be administered to these patients only after benefit-risk assessment. Use in women Sildenafil is not indicated for use by women. Warnings relating to excipients This medicinal product contains 1 mg of sodium benzoate (E211) in each millilitre of suspension.
Effects on Driving
4.7 Effects on ability to drive and use machines No studies have been conducted on ability to drive and use machines. As dizziness and altered vision were reported in clinical trials with sildenafil, patients should be aware of how they react to sildenafil, before driving or using machines.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
