Quest for the right Drug
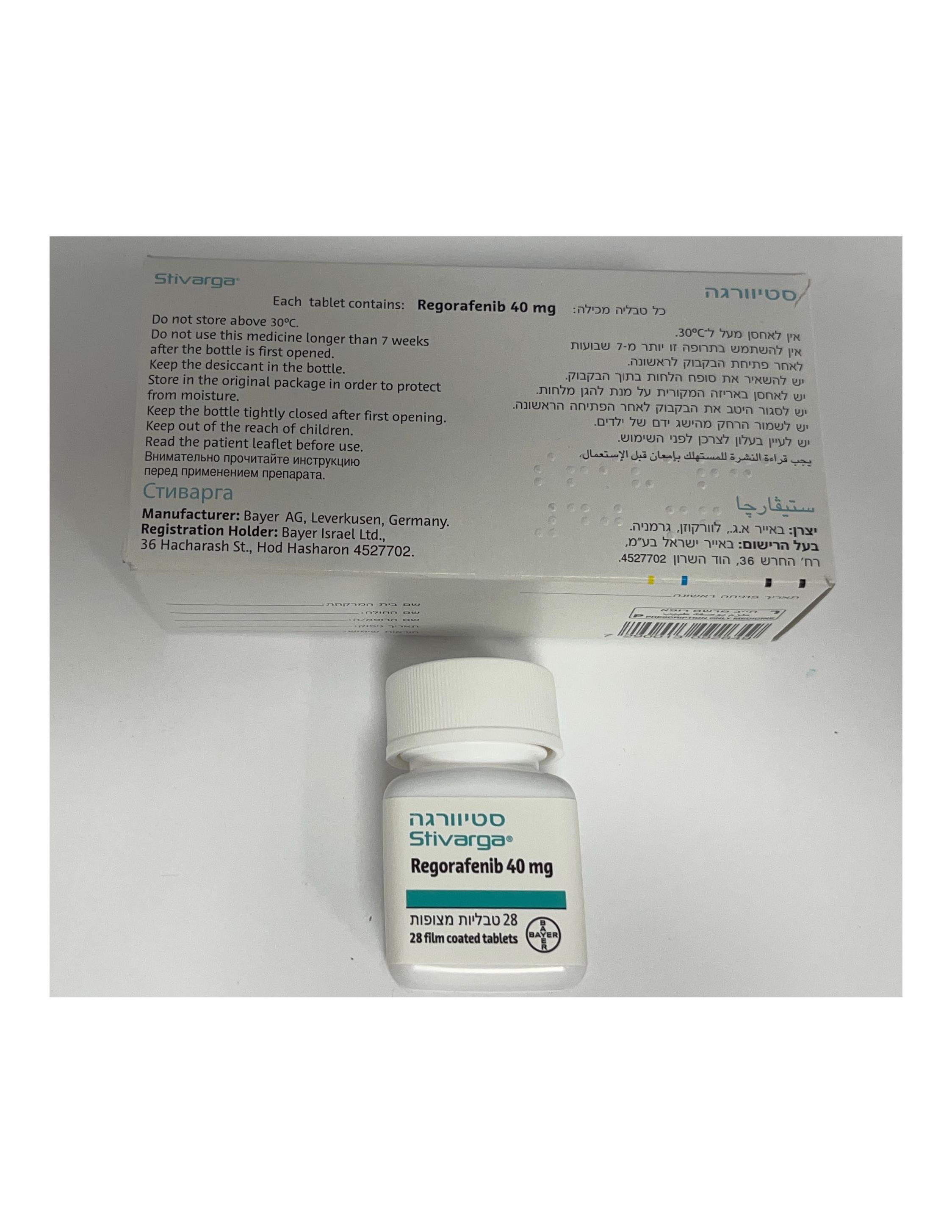
סטיוורגה STIVARGA (REGORAFENIB)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות מצופות פילם : FILM COATED TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Interactions : אינטראקציות
4.5 Interaction with other medicinal products and other forms of interaction Inhibitors of CYP3A4 and UGT1A9 / inducers of CYP3A4 In vitro data indicate that regorafenib is metabolized by cytochrome CYP3A4 and uridine diphosphate glucuronosyl transferase UGT1A9. Administration of ketoconazole (400 mg for 18 days), a strong CYP3A4 inhibitor, with a single dose of regorafenib (160 mg on day 5) resulted in an increase in mean exposure (AUC) of regorafenib of approximately 33%, and a decrease in mean exposure of the active metabolites, M-2 (N-oxide) and M-5 (N-oxide and N-desmethyl), of approximately 90%. It is recommended to avoid concomitant use of strong inhibitors of CYP3A4 activity (e.g. clarithromycin, grapefruit juice, itraconazole, ketoconazole, posaconazole, telithromycin and voriconazole) as their influence on the steady-state exposure of regorafenib and its metabolites has not been studied. Co-administration of a strong UGT1A9 inhibitor (e.g. mefenamic acid, diflunisal, and niflumic acid) during regorafenib treatment should be avoided, as their influence on the steady-state exposure of regorafenib and its metabolites has not been studied. Administration of rifampicin (600 mg for 9 days), a strong CYP3A4 inducer, with a single dose of regorafenib (160 mg on day 7) resulted in a reduction in AUC of regorafenib of approximately 50%, a 3- to 4-fold increase in mean exposure of the active metabolite M-5, and no change in exposure of active metabolite M-2. Other strong CYP3A4 inducers (e.g. phenytoin, carbamazepine, phenobarbital and St. John’s wort) may also increase metabolism of regorafenib. Strong inducers of CYP3A4 should be avoided, or selection of an alternate concomitant medicinal product, with no or minimal potential to induce CYP3A4 should be considered. UGT1A1 and UGT1A9 substrates In vitro data indicate that regorafenib as well as its active metabolite M-2 inhibit glucuronidation mediated by UGT1A1 and UGT1A9 whereas M-5 only inhibits UGT1A1 at concentrations which are achieved in vivo at steady state. Administration of regorafenib with a 5-day break prior to administration of irinotecan resulted in an increase of approximately 44% in AUC of SN-38, a substrate of UGT1A1 and an active metabolite of irinotecan. An increase in AUC of irinotecan of approximately 28% was also observed. This indicates that co-administration of regorafenib may increase systemic exposure to UGT1A1 and UGT1A9 substrates. Breast cancer resistance protein (BCRP) and P-glycoprotein substrates Administration of regorafenib (160 mg for 14 days) prior to administration of a single dose of rosuvastatin (5 mg), a BCRP substrate, resulted in a 3.8-fold increase in mean exposure (AUC) of rosuvastatin and a 4.6-fold increase in Cmax. This indicates that co-administration of regorafenib may increase the plasma concentrations of other concomitant BCRP substrates (e.g. methotrexate, fluvastatin, atorvastatin). Therefore, it is recommended to monitor patients closely for signs and symptoms of increased exposure to BCRP substrates. Clinical data indicate that regorafenib has no effect on digoxin pharmacokinetics, therefore can be given concomitantly with p-glycoprotein substrates, such as digoxin, without a clinically meaningful drug interaction. Inhibitors of P-glycoprotein and BCRP / Inducers of P-glycoprotein and BCRP In vitro studies indicate that the active metabolites M-2 and M-5 are substrates for P-glycoprotein and BCRP. Inhibitors and inducers of BCRP and P-glycoprotein may interfere with the exposure of M-2 and M-5. The clinical significance of these findings is unknown (see also section 5.2). CYP isoform-selective substrates In vitro data indicate that regorafenib is a competitive inhibitor of the cytochromes CYP2C8 (Ki value of 0.6 micromolar), CYP2C9 (Ki value of 4.7 micromolar), CYP2B6 (Ki value of 5.2 micromolar) at concentrations which are achieved in vivo at steady state (peak plasma concentration of 8.1 micromolar). The in vitro inhibitory potency towards CYP3A4 (Ki value of 11.1 micromolar) and CYP2C19 (Ki value of 16.4 micromolar) was less pronounced. A clinical probe substrate study was performed to evaluate the effect of 14 days of dosing with 160 mg regorafenib on the pharmacokinetics of probe substrates of CYP2C8 (rosiglitazone) CYP2C9 (S-warfarin), CYP 2C19 (omeprazole) and CYP3A4 (midazolam). Pharmacokinetic data indicate that regorafenib may be given concomitantly with substrates of CYP2C8, CYP2C9, CYP3A4, and CYP2C19 without a clinically meaningful drug interaction (see also section 4.4). Antibiotics The concentration-time profile indicates that regorafenib and its metabolites may undergo enterohepatic circulation (see section 5.2). Co-administration with neomycin, a poorly absorbed antimicrobial agent used for eradicating the gastrointestinal microflora (which may interfere with the enterohepatic circulation of regorafenib) had no effect on the regorafenib exposure, but there was an approximately 80% decrease in the exposure of the active metabolites M-2 and M-5 which showed in vitro and in vivo comparable pharmacological activity as regorafenib. The clinical significance of this neomycin interaction is unknown, but may result in a decreased efficacy of regorafenib. Pharmacokinetic interactions of other antibiotics have not been studied. Bile salt-sequestering agents Regorafenib, M-2 and M-5 are likely to undergo enterohepatic circulation (see section 5.2). Bile salt- sequestering agents such as cholestyramine and cholestagel may interact with regorafenib by forming insoluble complexes which may impact absorption (or reabsorption), thus resulting in potentially decreased exposure. The clinical significance of these potential interactions is unknown, but may result in a decreased efficacy of regorafenib.

פרטי מסגרת הכללה בסל
א. התרופה תינתן לטיפול בסרקומה מסוג GIST לחולים שמחלתם התקדמה לאחר טיפול בשני מעכבי טירוזין קינאז (Imatinib, Sunitinib). ב. מתן התרופה האמורה ייעשה לפי מרשם של רופא מומחה באונקולוגיה.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| התרופה תינתן לטיפול בסרקומה מסוג GIST לחולים שמחלתם התקדמה לאחר טיפול בשני מעכבי טירוזין קינאז |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
15/01/2015
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
21.01.16 - עלון לצרכן 17.05.16 - עלון לצרכן 15.03.18 - עלון לצרכן 10.08.22 - עלון לצרכן אנגלית 10.08.22 - עלון לצרכן עברית 10.08.22 - עלון לצרכן ערבית 18.09.22 - עלון לצרכן עברית 27.02.23 - עלון לצרכן אנגלית 05.06.23 - עלון לצרכן עברית 27.02.23 - עלון לצרכן ערבית 31.07.23 - עלון לצרכן אנגלית 31.07.23 - עלון לצרכן עברית 31.07.23 - עלון לצרכן ערבית 02.08.15 - החמרה לעלון 26.09.16 - החמרה לעלון 25.04.17 - החמרה לעלון 04.12.18 - החמרה לעלון 03.02.20 - החמרה לעלון 26.04.20 - החמרה לעלון 18.11.21 - החמרה לעלון 18.09.22 - החמרה לעלון 05.06.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
סטיוורגה