Quest for the right Drug
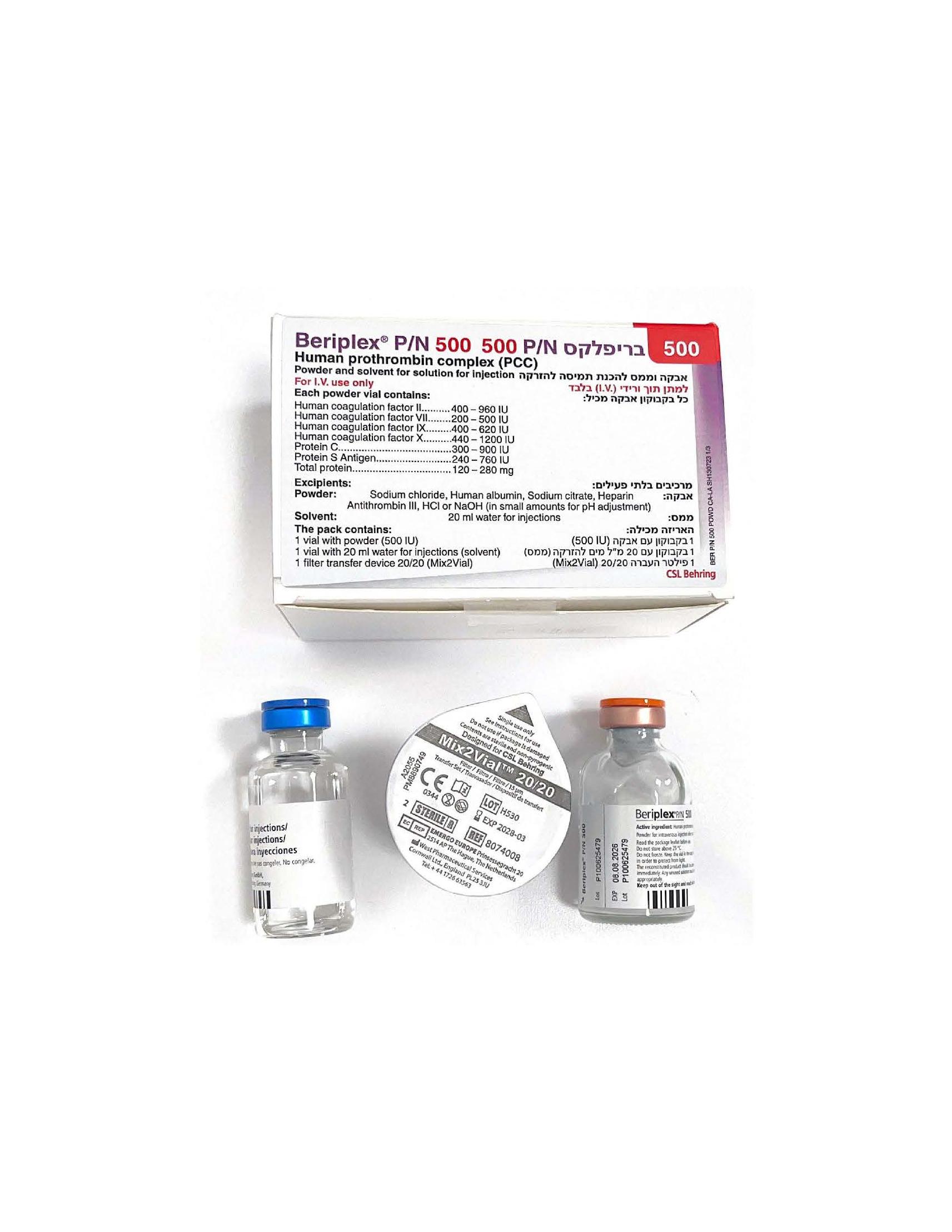
בריפלקס P/N 500 BERIPLEX ® P/N 500 (COAGULATION FACTOR II (HUMAN), COAGULATION FACTOR IX (HUMAN), COAGULATION FACTOR IX (HUMAN- RFIXFC), COAGULATION FACTOR VII (HUMAN), COAGULATION FACTOR X (HUMAN), PROTEIN C, PROTEIN S ANTIGEN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה וממס להכנת תמיסה להזרקה : POWDER AND SOLVENT FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the Safety Profile Allergic or anaphylactic-type reactions have been uncommonly observed, including severe anaphylactic reactions (see section 4.4). Replacement therapy may lead to the formation of circulating antibodies inhibiting one or more of the human prothrombin complex factors. If such inhibitors occur, the condition will manifest itself as a poor clinical response. In such cases, it is recommended to contact a specialised haemophilia center for guidance. Anaphylactic reactions have been observed in patients with antibodies to factors contained in Beriplex. Increase in body temperature has been commonly observed. There is a risk of thromboembolic episodes following the administration of human prothrombin complex (see section 4.4). Tabulated list of adverse drug reactions of Beriplex The following adverse reactions are based on clinical trial data, post marketing experience as well as scientific literature. The table presented below is according to the MedDRA system organ classification (SOC and Preferred Term Level). Frequencies have been based on clinical trial data, according to the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000) or not known (cannot be estimated from the available data). MedDRA Standard Adverse Drug Reaction by PT Frequency System Organ Class Vascular disorders and Thromboembolic events* common other SOCs MedDRA Standard Adverse Drug Reaction by PT Frequency System Organ Class Blood and lymphatic Disseminated intravascular not known system disorders coagulation Immune system Hypersensitivity or allergic uncommon disorders reactions Anaphylactic reactions including not known anaphylactic shock Development of antibodies not known Nervous system Headache common disorders General disorders and Body temperature increased common administration site conditions *including cases with fatal outcome For safety with respect to transmissible agents, see section 4.4. Paediatric population No data are available regarding the use of Beriplex in paediatric population. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/ and emailed to the Registration Holder’s Patient Safety Unit at: PV-IL@cslbehring.com

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
