Quest for the right Drug
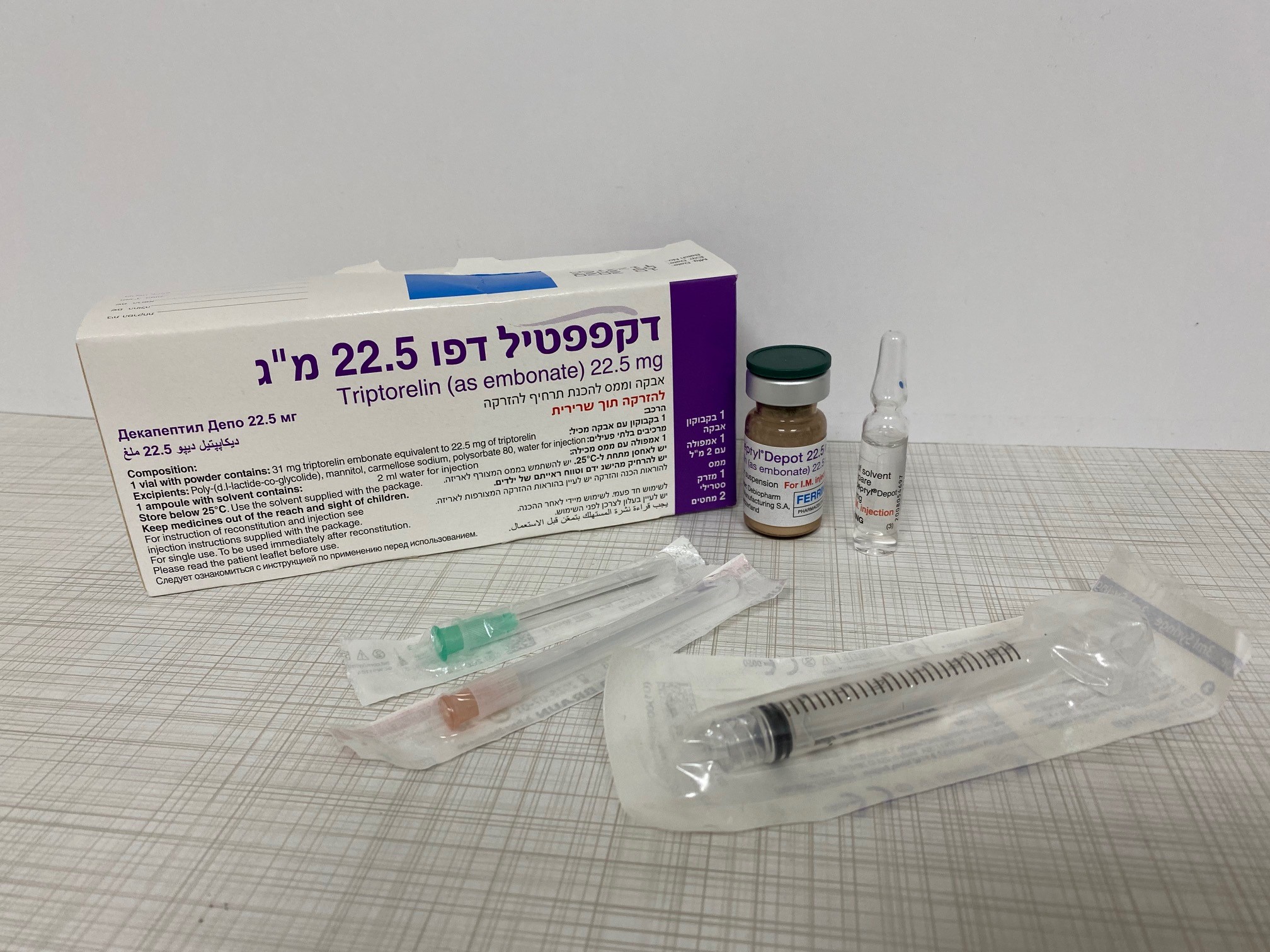
דקפפטיל דפו 22.5 מ"ג DECAPEPTYL DEPOT 22.5 MG (TRIPTORELIN AS EMBONATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
אבקה וממס להכנת תרחיף להזרקה : POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1Pharmacodynamic properties Pharmacotherapeutic group: Hormones and related agents, gonadotropin releasing hormone analogues. ATC code: L02AE04 Mechanism of action and pharmacodynamic effects Triptorelin, a GnRH agonist, acts as a potent inhibitor of gonadotrophin secretion when given continuously and in therapeutic doses. Animal and human studies show that after administrationof triptorelin there is an initial and transient increase in circulating levels of luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone in males and oestradiol in females. However, chronic and continuous administration of triptorelin results in decreased LH and FSH secretion and suppression of testicular and ovarian steroidogenesis. In men with prostate cancer: A reduction of serum testosterone levels into the range normally seen in surgically castrated men occurs approximately 2 to 4 weeks after initiation of therapy. Decapeptyl depot 22.5 mg is designed todeliver 22.5 mg of triptorelin over a 6-month period. Once the castration levels of testosterone have been achieved by the end of the first month, serum testosterone levels are maintained for aslong as the patients receive their injection every twenty-four weeks. This results in accessory sexual organ atrophy. These effects are generally reversible upon discontinuation of the medicinal product. The effectiveness of treatment can be monitored by measuring serum levels of testosterone and prostate specific antigen. As shown during the clinicaltrial programme, there was a 97% median relative reduction in PSA at Month 6 for Decapeptyl depot 22.5 mg. In animals, administration of triptorelin resulted in the inhibition of growth of some hormone- sensitive prostate tumours in experimental models. Clinical efficacy and safety in prostate cancer Administration of Decapeptyl depot 22.5 mg to patients with advanced prostate cancer as an intramuscular injection for a total of 2 doses (12 months) resulted in both achievement of castration levels of testosterone in 97.5% of patients after four weeks and maintenance of castration levels of testosterone in 93.0% of the patients from Month 2 through Month 12 of treatment. Clinical efficacy and safety in children with precocious puberty In a non-comparative clinical study, 44 children with central precocious puberty (39 girls and 5 boys) were treated with a total of two intramuscular injections of Decapeptyl 22.5 mg over 12 months (48 weeks). Suppression of stimulated LH concentrations to prepubertal levels was achieved in 95.5% of subjects by month 3, and in 93.2 % and 97.7% of subjects at months 6 and 12, respectively. The consequence is a regression or stabilisation of secondary sex characteristics and slowing down of accelerated bone maturation and growth. In girls, initial ovarian stimulation at treatment initiation, followed by the treatment-induced oestrogen increase, may lead, in the first month, to uterine ‘withdrawal’ bleeding of mild or moderate intensity.
Pharmacokinetic Properties
5.2. Pharmacokinetic properties Absorption: Following a single IM injection of Decapeptyl Depot 22. 5mg in patients with prostate cancer, tmax was 3 (2-12) hours and Cmax (0-169 days) was 39.9 (19.1-107.0)ng/ml. Triptorelin did not accumulate over 12 months of treatment. Distribution: Results of pharmacokinetic investigations conducted in healthy men indicate that after intravenous bolus administration, triptorelin is distributed and eliminated according to a 3- compartment model; and corresponding half-lives are approximately 6 minutes, 45 minutes, and 3 hours. The volume of distribution at steady state of triptorelin following intravenous administration of 0.5 mg triptorelin acetate is approximately 30 l in healthy male volunteers. Since there is no evidence that triptorelin at clinically relevant concentrations binds to plasma proteins, medicinal product interactions involving binding-site displacement are unlikely. Biotransformation: Metabolites of triptorelin have not been determined in humans. However, human pharmacokinetic data show that C-terminal fragments produced by tissue degradation are either completely destroyed within tissues or are rapidly further degraded in plasma or cleared by the kidneys. Elimination: Triptorelin is eliminated by both the liver and kidneys. Following intravenous injection of 0.5 mg triptorelin, 42 % of the dose was excreted in urine in the form of intact triptorelin. which increased to 62 % in subjects with hepatic impairment. Since creatinine clearance (Clcreat) in healthy volunteers was 150 ml/min and only 90 ml/min in subjects with hepatic impairment, this indicates that the liver is a major site of triptorelin elimination. In these healthy volunteers, the true terminal half-life of triptorelin was 2.8 hours and total clearance of triptorelin 212 ml/min, the latter being dependent on a combination of hepatic and renal elimination. Other special populations: Following intravenous administration of 0.5 mg triptorelin to subjects with moderate renal insufficiency (Clcreat 40 ml/min), triptorelin had an elimination half-life of 6.7 hours, 7.81 hours in subjects with severe renal insufficiency (Clcreat 8.9 ml/min) and 7.65 hours in patients with impaired hepatic function (Clcreat 89.9 ml/min). The effects of age and race on triptorelin pharmacokinetics have not been systematically studied.However, pharmacokinetic data obtained in young healthy male volunteers aged 20 to 22 years with an elevated creatinine clearance (approximately 150 ml/min) indicated that triptorelin was eliminated twice as fast in the young population. This is related to the fact that triptorelin clearanceis correlated to total creatinine clearance, which is well known to decrease with age. Because of the large safety margin of triptorelin and since Decapeptyl depot 22.5 mg is a sustained release formulation, no dose adjustment is recommended in patients with renal or hepaticimpairment. Pharmacokinetic/pharmacodynamic relationship The pharmacokinetics/pharmacodynamics relationship of triptorelin is not straightforward to assess, since it is non-linear and time-dependent. Thus, after acute administration in naive subjects, triptorelin induces a dose-dependent increase of LH and FSH responses. When administered as a sustained release formulation, triptorelin stimulates LH and FSH secretion during the first days post dosing and, in consequence, testosterone secretion. As shown by the results of the different bioequivalence studies, the maximal increase in testosterone is reached after around 4 days with an equivalent Cmax which is independent from the release rate oftriptorelin. This initial response is not maintained despite continuous exposure to triptorelin and is followed by a progressive and equivalent decrease of testosterone levels. In this case too, the extent of triptorelin exposure can vary markedly without affecting the overall effect on testosterone serum levels.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול במקרים האלה:1. הפחתת הורמוני מין בגברים פדופילים;2. הפחתת הורמוני מין בגברים הסובלים מפאראפיליות הכרוכות בדחף מיני מוגבר, עיסוק אינטניסיבי בפנטזיות ובדחפים מיניים סוטים, עד כדי פגיעה משמעותית בתפקוד ובסיכון מוחשי לזולת;הטיפול בתכשיר יינתן על פי מרשם של רופא מומחה בפסיכיאטריה במסגרת ליווי קבוע במרפאה לבריאות הנפש.3. סרטן הערמונית;4. אנדומטריוזיס;5. UTERUS MYOMATOSUS;6. טיפולי פוריות.מתן טיפול ב-Triptorelin לפדופילים או פאראפילים יינתן ללא השתתפות עצמית
שימוש לפי פנקס קופ''ח כללית 1994
יירשם ע"י רופא אורולוג, אונקולוג או רופא מורשה לחתום על מרשמי Pergonal. אושר ל-uterus leiomyoma ו-endometrial ablation רק כהכנה לניתוח ולא לטיפול ארוך טווח. אושר ל-endometriosis רק במקרים שזה מהווה בעיה לפוריות האישה או במקרים של תופעות לוואי מוכחות כתוצאה משימוש Danazol
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
09.08.22 - עלון לצרכן אנגלית 09.08.22 - עלון לצרכן עברית 09.08.22 - עלון לצרכן ערבית 08.06.23 - עלון לצרכן עברית 23.08.23 - עלון לצרכן אנגלית 23.08.23 - עלון לצרכן עברית 23.08.23 - עלון לצרכן ערבית 06.02.24 - עלון לצרכן עברית 28.03.24 - עלון לצרכן אנגלית 28.03.24 - עלון לצרכן עברית 28.03.24 - עלון לצרכן ערבית 08.06.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
דקפפטיל דפו 22.5 מ"ג