Quest for the right Drug
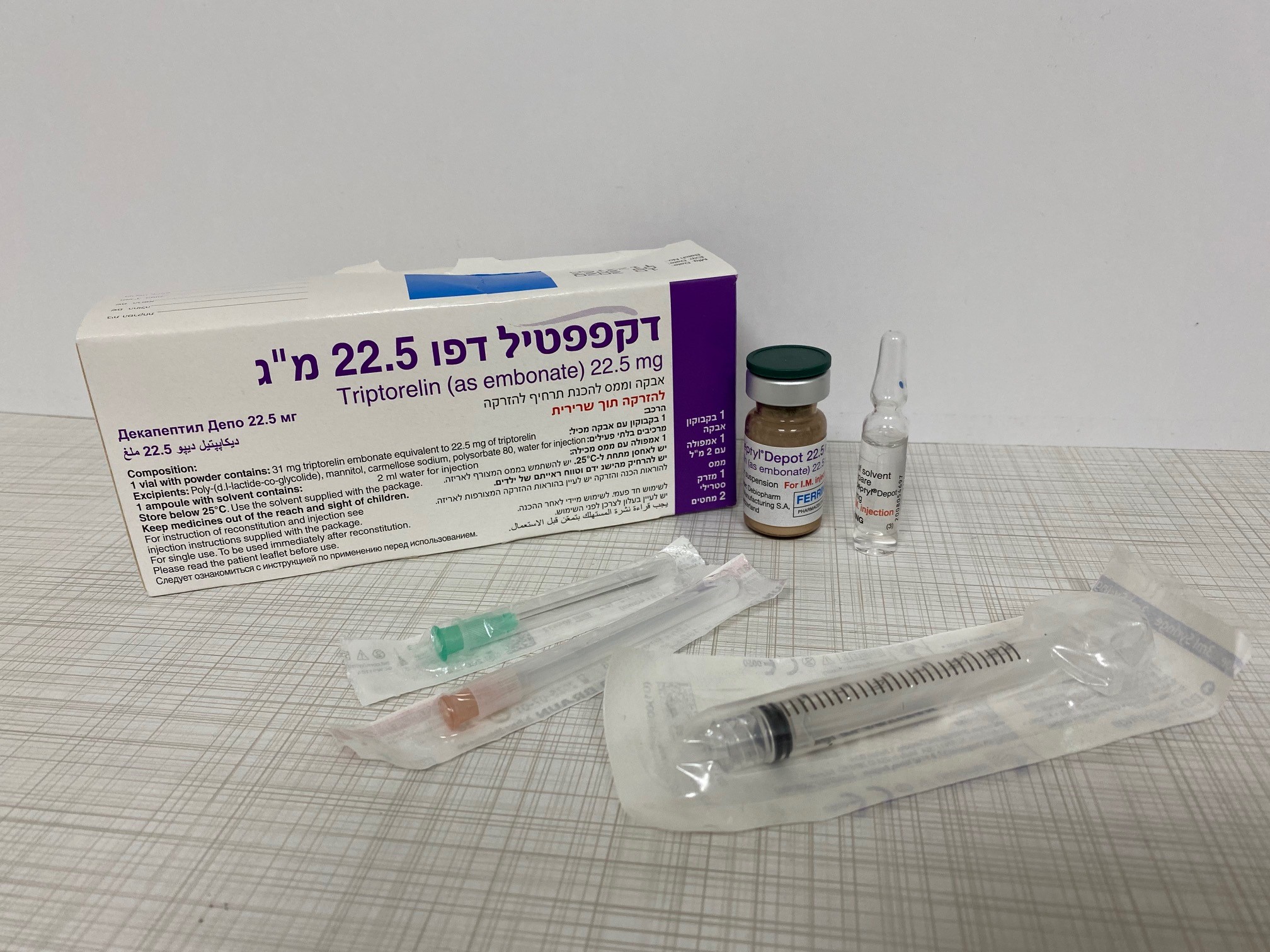
דקפפטיל דפו 22.5 מ"ג DECAPEPTYL DEPOT 22.5 MG (TRIPTORELIN AS EMBONATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
אבקה וממס להכנת תרחיף להזרקה : POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use The use of GnRH agonists may cause reduction in bone mineral density. In men, preliminary datasuggest that the use of a bisphosphonate in combination with an GnRH agonist may reduce bonemineral loss. Particular caution is necessary in patients with additional risk factors for osteoporosis (e.g. chronic alcohol abuse, smokers, long-term therapy with drugs that reduce bonemineral density, e.g. anticonvulsants or corticoids, family history of osteoporosis, malnutrition). Rarely, treatment with GnRH agonists may reveal the presence of a previously unknown gonadotroph cell pituitary adenoma. These patients may present with a pituitary apoplexy characterised by sudden headache, vomiting, visual impairment and ophthalmoplegia. There is an increased risk of incident depression (which may be severe) in patients undergoing treatment with GnRH agonists, such as triptorelin. Patients should be informed accordingly and treated appropriately if symptoms occur. Patients with known depression should be monitored closely during therapy. Caution is required with intramuscular injection in patients treated with anticoagulants, due to the potential risk of haematomas at the site of injection. The efficacy and safety of Decapeptyl depot 22.5 mg has been established via intramuscular route only. Subcutaneous administration is not recommended. This medicine contains less than 1 mmol (23mg) sodium per dose, i.e., essentially “sodium- free”. Prostate Cancer: Initially, triptorelin, like other GnRH agonists, causes a transient increase in serum testosterone levels. As a consequence, isolated cases of transient worsening of signs and symptoms of prostate cancer may occasionally develop during the first weeks of treatment. During the initial phase of treatment, consideration should be given to the additional administration of a suitable anti- androgen to counteract the initial rise in serum testosterone levels and the worsening of clinical symptoms. A small number of patients may experience a temporary worsening of signs and symptoms of their prostate cancer (tumour flare) and a temporary increase in cancer related pain (metastatic pain), which can be managed symptomatically. As with other GnRH agonists, isolated cases of spinal cord compression or urethral obstruction have been observed. If spinal cord compression or renal impairment develops, standard treatmentof these complications should be instituted, and in extreme cases an immediate orchiectomy (surgical castration) should be considered. Careful monitoring is indicated during the first weeksof treatment, particularly in patients suffering from vertebral metastases, at the risk of spinal cordcompression, and in patients with urinary tract obstruction. After surgical castration, triptorelin does not induce any further decrease in serum testosterone levels. Once castration levels of testosterone have been achieved by the end of the first month, serum testosterone levels are maintained for as long as the patients receive their injection every 6months (twenty-four weeks). The effectiveness of treatment can be monitored by measuring serum levels of testosterone and prostate specific antigen. Long-term androgen deprivation either by bilateral orchiectomy or administration of GnRH analogues is associated with increased risk of bone loss and may lead to osteoporosis and increased risk of bone fracture. Androgen deprivation therapy may prolong the QT interval. In patients with a history of or risk factors for QT prolongation and in patients receiving concomitant medicinal products that might prolong the QT interval (see section 4.5) physicians should assess the benefit/risk profile including the potential for Torsades de pointes prior to initiating Decapeptyl depot 22.5 mg. In addition, from epidemiological data, it has been observed that patients may experience metabolic changes (e.g. glucose intolerance, fatty liver), or an increased risk of cardiovascular disease during androgen deprivation therapy. However, prospective data did not confirm the link between treatment with GnRH analogues and an increase in cardiovascular mortality. Patients at high riskfor metabolic or cardiovascular diseases should be carefully assessed before commencing treatment and adequately monitored during androgen deprivation therapy. Administration of triptorelin in therapeutic doses results in suppression of the pituitary gonadal system. Normal function is normally restored after treatment is discontinued. The results of diagnostic tests of pituitary gonadal function conducted during treatment and after discontinuation of therapy with GnRH analogues may therefore be misleading. Precocious puberty Treatment of children with progressive brain tumours should follow a careful individual appraisal of the risks and benefits. Pseudo-precocious puberty (gonadal or adrenal tumour or hyperplasia) and gonadotropin- independent precocious puberty (testicular toxicosis, familial Leydig cell hyperplasia) should be precluded. In girls, initial ovarian stimulation at treatment initiation, followed by the treatment-induced oestrogen withdrawal, may lead, in the first month, to vaginal bleeding of mild or moderate intensity. The therapy is a long-term treatment, adjusted individually. Decapeptyl depot 22.5mg should be administered as precisely as possible in regular 6 monthly periods. An exceptional delay of the injection date for a few days (169 ± 3 days) does not influence the results of the therapy. After discontinuation of treatment the development of puberty characteristics will occur. Information with regards to future fertility is still limited but future reproductive function and fertility appears to be unaffected by GnRH treatment. In most girls, regular menses will start on average one year after ending the therapy. Bone mineral density may decrease during GnRH agonist therapy for central precocious puberty due to the expected effects of oestrogen suppression. However, after cessation of treatment subsequent bone mass accrual is preserved and peak bone mass in late adolescence does not seem to be affected by treatment. Slipped capital femoral epiphysis can be seen after withdrawal of GnRH agonist treatment. The suggested theory is that the low concentrations of oestrogen during treatment with GnRH agonists weaken the epiphysial plate. The increase in growth velocity after stopping the treatment subsequently results in a reduction of the shearing force needed for displacement of the epiphysis. Idiopathic intracranial hypertension Idiopathic intracranial hypertension (pseudotumor cerebri) has been reported in paediatric patients receiving triptorelin. Patients should be warned for signs and symptoms of idiopathic intracranial hypertension, including severe or recurrent headache, vision disturbances and tinnitus. If idiopathic intracranial hypertension occurs, discontinuation of triptorelin should be considered.
Effects on Driving
4.7 Effects on ability to drive and use machines No studies on the effects on the ability to drive and use machines have been performed. However,the ability to drive and use machines may be impaired should the patient experience dizziness, somnolence and visual disturbances being possible undesirable effects of treatment, or resulting from the underlying disease.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול במקרים האלה:1. הפחתת הורמוני מין בגברים פדופילים;2. הפחתת הורמוני מין בגברים הסובלים מפאראפיליות הכרוכות בדחף מיני מוגבר, עיסוק אינטניסיבי בפנטזיות ובדחפים מיניים סוטים, עד כדי פגיעה משמעותית בתפקוד ובסיכון מוחשי לזולת;הטיפול בתכשיר יינתן על פי מרשם של רופא מומחה בפסיכיאטריה במסגרת ליווי קבוע במרפאה לבריאות הנפש.3. סרטן הערמונית;4. אנדומטריוזיס;5. UTERUS MYOMATOSUS;6. טיפולי פוריות.מתן טיפול ב-Triptorelin לפדופילים או פאראפילים יינתן ללא השתתפות עצמית
שימוש לפי פנקס קופ''ח כללית 1994
יירשם ע"י רופא אורולוג, אונקולוג או רופא מורשה לחתום על מרשמי Pergonal. אושר ל-uterus leiomyoma ו-endometrial ablation רק כהכנה לניתוח ולא לטיפול ארוך טווח. אושר ל-endometriosis רק במקרים שזה מהווה בעיה לפוריות האישה או במקרים של תופעות לוואי מוכחות כתוצאה משימוש Danazol
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
09.08.22 - עלון לצרכן אנגלית 09.08.22 - עלון לצרכן עברית 09.08.22 - עלון לצרכן ערבית 08.06.23 - עלון לצרכן עברית 23.08.23 - עלון לצרכן אנגלית 23.08.23 - עלון לצרכן עברית 23.08.23 - עלון לצרכן ערבית 06.02.24 - עלון לצרכן עברית 28.03.24 - עלון לצרכן אנגלית 28.03.24 - עלון לצרכן עברית 28.03.24 - עלון לצרכן ערבית 08.06.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
דקפפטיל דפו 22.5 מ"ג