Quest for the right Drug
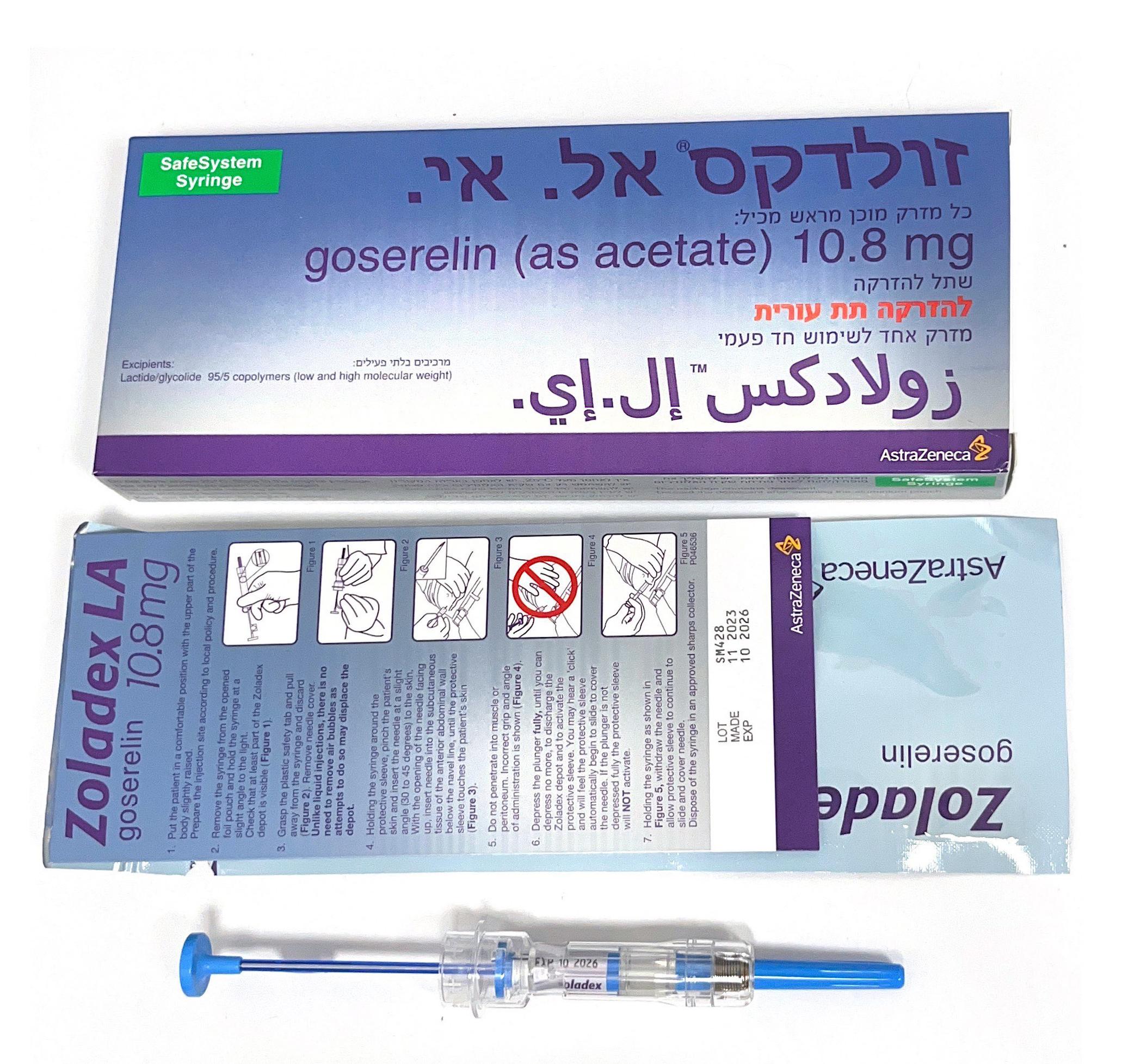
זולדקס אל.אי. ZOLADEX LA (GOSERELIN AS ACETATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
שתל להזרקה : IMPLANT FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The following frequency categories for adverse drug reactions (ADRs) were calculated based on reports from ZOLADEX clinical trials and post-marketing sources. The most commonly observed adverse reactions include hot flushes, sweating and injection site reactions. The following convention has been used for classification of frequency: Very common (≥1/10), Common (≥1/100 to <1/10), Uncommon (≥1/1,000 to <1/100), Rare (≥ 1/10,000 to <1/1,000), Very rare (<1/10,000) and Not known (cannot be estimated from the available data). Table: ZOLADEX LA adverse drug reactions presented by MedDRA System Organ Class SOC Frequency Adverse reaction Neoplasms benign, malignant and Very rare Pituitary tumour unspecified (including cysts and polyps) Page 4 of 10 5 Blood and lymphatic system disorders Not knownh Anaemia, Leucopenia and Thrombocytopenia Immune system disorders Uncommon Drug hypersensitivity Rare Anaphylactic reaction Endocrine disorders Very rare Pituitary haemorrhage Metabolism and nutrition disorders Common Glucose tolerance impaireda Psychiatric disorders Very common Libido decreasedb Common Mood changes, depression Very rare Psychotic disorder Nervous system disorders Common Paraesthesia Spinal cord compression Not known Memory impairment Cardiac disorders Common Cardiac failuref, myocardial infarctionf Not known QT prolongation (see sections 4.4 and 4.5) Vascular disorders Very common Hot flushb Common Blood pressure abnormalc Not knownh Pulmonary embolism Hepatobiliary disorders Not knownh Hepatic dysfunction and Jaundice Skin and subcutaneous tissue Very common Hyperhidrosisb disorders Common Rashd Not known Alopeciag Musculoskeletal, connective tissue and Common Bone paine bone disorders Uncommon Arthralgia Respiratory, thoracic and mediastinal Not knownh Interstitial lung disease Renal and urinary disorders Uncommon Ureteric obstruction Reproductive system and breast Very common Erectile dysfunction disorders Common Gynaecomastia Uncommon Breast tenderness General disorders and administration Common Injection site reaction site conditions Not knownh Tumour flare (on initiation of treatment) Page 5 of 10 6 Investigations Common Bone density decreased (see section 4.4), weight increased a A reduction in glucose tolerance has been observed in males receiving LHRH agonists. This may manifest as diabetes or loss of glycaemic control in those with pre-existing diabetes mellitus. b These are pharmacological effects which seldom require withdrawal of therapy. Hyperhidrosis and hot flushes may continue after stopping Zoladex. c These may manifest as hypotension or hypertension, have been occasionally observed in patients administered Zoladex. d These are generally mild, often regressing without discontinuation of therapy. e Initially, prostate cancer patients may experience a temporary increase in bone pain, which can be managed symptomatically. f Observed in a pharmaco-epidemiology study of LHRH agonists used in the treatment of prostate cancer. The risk appears to be increased when used in combination with anti-androgens. g Particularly loss of body hair, an expected effect of lowered androgen levels. h Frequency of the adverse drug reactions is based on spontaneous data. Description of selected adverse event Blood pressure abnormal: The changes are usually transient, resolving either during continued therapy or after cessation of therapy with Zoladex. Rarely, such changes have been sufficient to require medical intervention, including withdrawal of treatment from Zoladex. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| Breast cancer for premenopausal women | אונקולוגיה | GOSERELIN, LEUPRORELIN |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
09/03/1999
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
