Quest for the right Drug
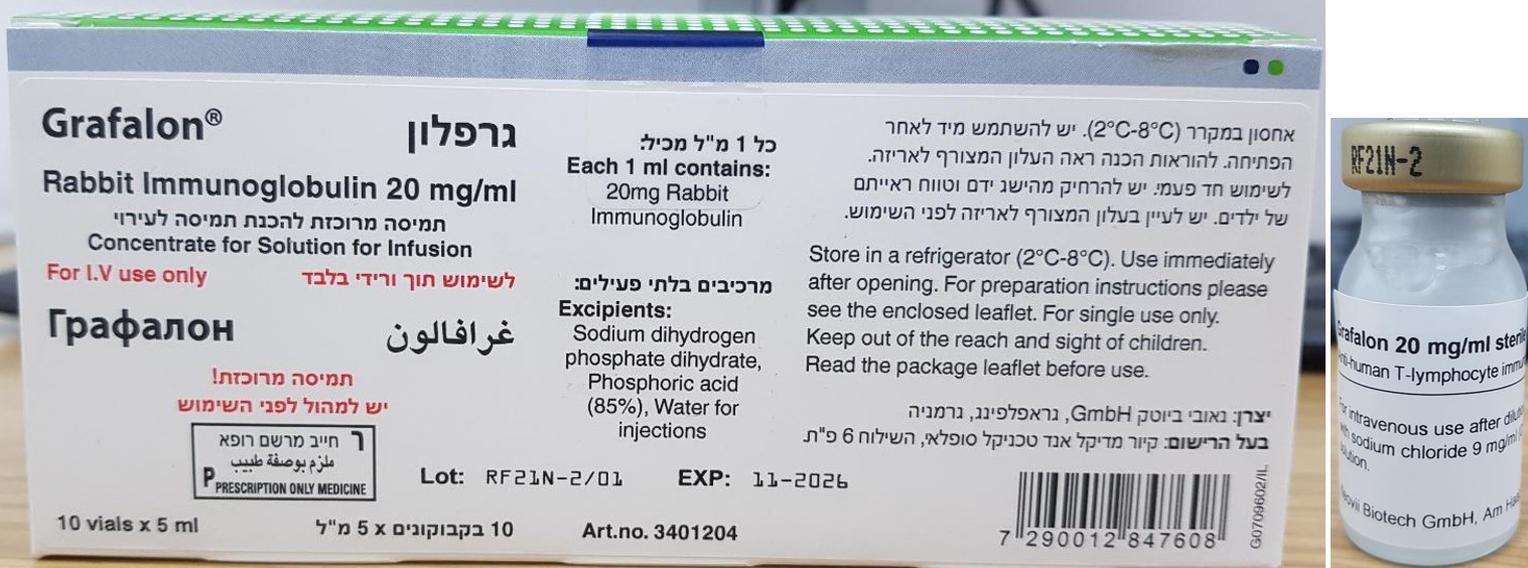
גרפלון GRAFALON (RABBIT IMMUNOGLOBULIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תרכיז להכנת תמיסה לאינפוזיה : CONCENTRATE FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile Grafalon is an immunoglobulin product with immunosuppressive properties. Well-known class-related adverse effects include cytokine-release related symptoms, hypersensitivity reactions such as anaphylaxis and other allergic phenomena, enhanced susceptibility to infections, and occurrence of malignancies. The nature and frequency of adverse reactions described in this section were analysed in an integrated safety analysis on the basis of 6 clinical studies consisting of 242 patients in the indications prevention of rejection in patients receiving renal transplants (136 patients) and conditioning prior to allogeneic stem cell transplantation (106 patients). Approx. 94% of the patients analysed experienced at least one adverse reaction. The pattern of adverse reactions reflects in part common complications typically occurring after the respective procedures - renal transplantation (urinary tract infection, renal failure) and stem cell transplantation (pancytopenia, mucosal inflammation). In the table below, adverse reactions reported with Grafalon are listed and classified according to frequency and System Organ Class. Frequency groupings are defined according to the following convention: very common (1/10), common (1/100 to <1/10), uncommon (1/1,000 to <1/100). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness. Tabulated list of adverse reactions Infections and infestations Very common CMV infection*, urinary tract infection* Common bacterial sepsis**, pneumonia**, pyelonephritis*, herpes infection, influenza, oral candidiasis, bronchitis, rhinitis, sinusitis, nasopharyngitis, skin infection Uncommon catheter site infection, Epstein-Barr virus infection, gastrointestinal infection, erysipelas, wound infection Neoplasms benign, malignant and unspecified (incl cysts and polyps) Common lymphoproliferative disorder* Blood and lymphatic system disorders Very common anemia Common pancytopenia**, thrombocytopenia, leukopenia Uncommon polycythemia Immune system disorders Common anaphylactic shock**, anaphylactic reaction, hypersensitivity Metabolism and nutrition disorders Common hyperlipidemia Uncommon fluid retention, hypercholesterolemia Nervous system disorders Very common headache, tremor Common paresthesia Eye disorders Common photophobia Cardiac disorders Common tachycardia Vascular disorders Very common flushing Common hypotension*, venoocclusive disease, hypertension Uncommon shock**, lymphocele Respiratory, thoracic and mediastinal disorders Very common dyspnea Common cough, epistaxis Gastrointestinal disorders Very common vomiting, nausea, diarrhea, abdominal pain Common stomatitis Uncommon reflux esophagitis, dyspepsia Hepatobiliary disorders Common hyperbilirubinemia Skin and subcutaneous tissue disorders Common erythema, pruritus, rash Uncommon drug eruption Musculoskeletal and connective tissue disorders Common myalgia, arthralgia, back pain, musculoskeletal stiffness Renal and urinary disorders Common renal tubular necrosis*, hematuria Uncommon renal failure**, renal necrosis* General disorders and administration site conditions Very common pyrexia**, chills Common asthenia, chest pain, hyperthermia, mucosal inflammation, peripheral edema Uncommon edema Investigations Common blood creatinine increased*, Cytomegalovirus antigen positive, C-reactive protein increased Uncommon hepatic enzymes increased * serious reaction ** serious reaction, in single cases with fatal outcome Description of selected adverse reactions Cytokine release related symptoms These reactions occur due to release of cytokines and include fever, chills, headache, nausea, vomiting, tachycardia, and circulatory changes. These reactions could be summarized under the clinical entity of cytokine release syndrome. They are frequently observed during or after the administration of Grafalon. Symptoms are usually well manageable. Prophylactic medication could be administered to alleviate these symptoms. Hypersensitivity reactions Reactions such as flushing, rash, erythema, edema, dyspnea with or without bronchospasm, and cough are commonly observed during and after the administration. These reactions usually respond to treatment well. The administration of appropriate prophylactic medication can ameliorate these symptoms. The occurrence of anaphylaxis/anaphylactic shock requires immediate termination of the infusion. Serum sickness, observed when Grafalon is administered for long treatment duration and at lower dosage, is rarely severe and usually responds well to symptomatic treatment. Hematological changes Transient changes of thrombocyte and leukocyte counts, also known as thrombocytopenia and leukopenia are commonly observed after Grafalon administration. Anemia is also very commonly observed after administration of Grafalon. Infections The patients treated with immunosuppressive regimens have an increased susceptibility to infections. In the first year after solid organ transplantation, the majority of patients who received Grafalon developed infections of bacterial, viral or mycotic origin. Urinary tract infection is a very common bacterial infection; very common viral infections are caused by cytomegalovirus (CMV). Commonly reported infections include bacterial sepsis, bacterial pneumonia, pyelonephritis, herpetic viral infections, and oral candidiasis. EBV infections, CMV pneumonia and CMV gastroenteritis are uncommon viral infections. Systemic candidiasis is an uncommon fungal infection. The majority of infections are usually manageable with the respective treatment. There were isolated reports of life-threatening or even fatal infections. Appropriate monitoring and prophylactic treatment can reduce the infection rate. Malignancy The incidence of malignancy occurring after Grafalon treatment is generally low across studies and publications and is comparable with the incidence observed with other combinations of immunosuppressive medications. Post-transplant lymphoproliferative disease was reported exclusively from patients who underwent allogeneic stem cell transplantation (1.7%). Hemolysis Rare cases (less than 1 in every 1000 patients) of hemolysis were reported in connection with Grafalon administration and were fatal in isolated cases. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/ and emailed to the Registration Holder’s Patient Safety Unit at: drugsafety@neopharmgroup.com

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
