Quest for the right Drug
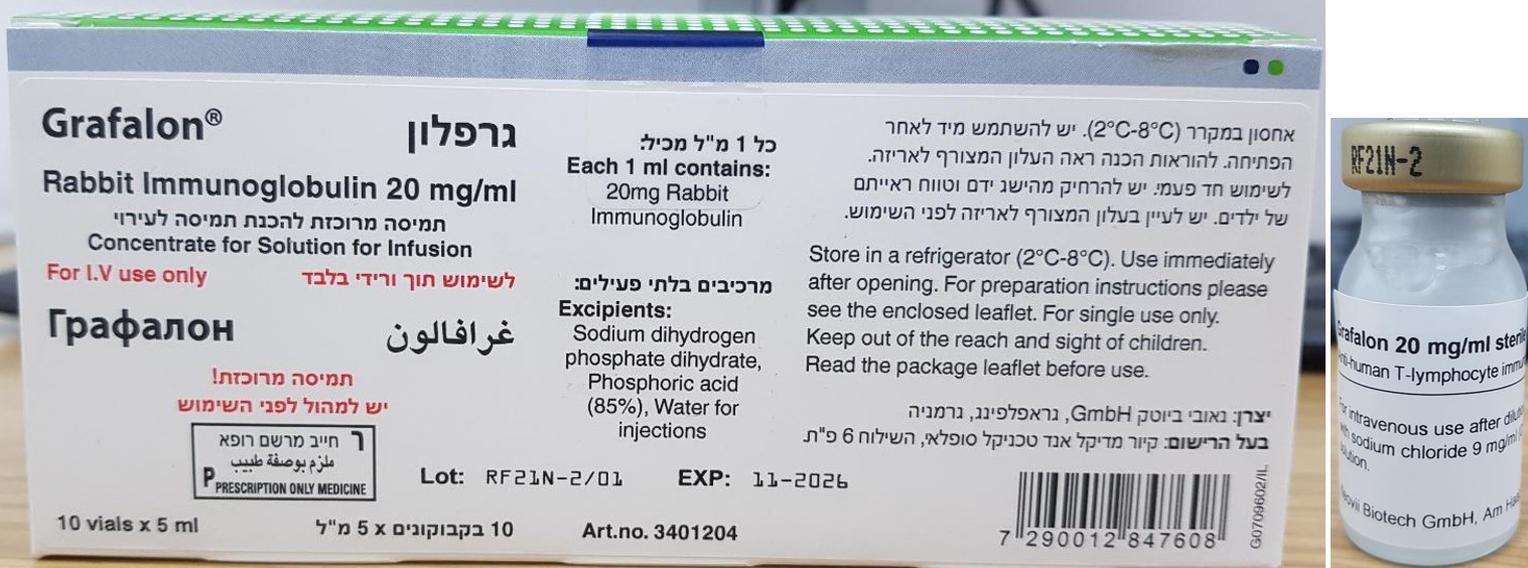
גרפלון GRAFALON (RABBIT IMMUNOGLOBULIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תרכיז להכנת תמיסה לאינפוזיה : CONCENTRATE FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Patients receiving Grafalon must be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources to provide emergency treatment if necessary. Grafalon must be administered and monitored under appropriately qualified medical supervision. Hypersensitivity reactions Hypersensitivity reactions have been reported with the administration of Grafalon. Before the first administration of Grafalon, it is recommended to determine whether the patient has an anamnestical allergic predisposition, in particular to rabbit proteins. In case of re-exposure in form of re-therapy with Grafalon or treatment with rabbit immunoglobulin preparations of other manufacturers, the risk of developing an anaphylactic reaction is increased due to a possible sensitisation during the former therapy. Severe thrombocytopenia Treatment with Grafalon should be interrupted or stopped in solid organ transplant patients in whom severe thrombocytopenia develops (i.e. less than 50,000 platelets/l) as Grafalon may enhance thrombocytopenia and thus increase the risk of hemorrhage. Clinical personnel should be prepared for appropriate emergency measures. Hepatic disorders Grafalon has to be administered with special caution in patients with hepatic diseases as it may aggravate pre-existing clotting disorders. Careful monitoring of thrombocytes and coagulation parameters is recommended. Cardiovascular disorders Grafalon has to be administered with special caution in patients with known or suspected cardiovascular disorders. In patients with hypotension or cardiac decompensation with orthostatic symptoms (e.g. unconsciousness, weakness, vomiting, nausea), slowing/interrupting the infusion should be considered. Infections Immunosuppressive therapy increases the risk for infections in general. Grafalon treated patients have an increased risk for the development of bacterial, viral, parasitic, and/or mycotic infections. Adequate monitoring and treatment measures are indicated. In patients undergoing stem cell transplantation, monitoring of CMV- and EBV-status and adequate pre-emptive therapy are recommended. Vaccination During treatment with Grafalon, patients should be advised that non-live vaccinations might be less efficacious. Live attenuated virus vaccination is contraindicated in immunosuppressed patients. Warning on transmissible agents Standard measures to prevent infections resulting from the use of medicinal products prepared by using human components include careful selection of donors, screening of individual donations for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared by using human components are administered, the possibility of transmitting infective agents cannot be totally excluded. This also applies to unknown or emerging viruses and other pathogens. The measures taken for Grafalon are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) and for the non-enveloped hepatitis A and parvovirus B19 viruses. Traceability In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.
Effects on Driving
4.7 Effects on ability to drive and use machines Not relevant.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
