Quest for the right Drug
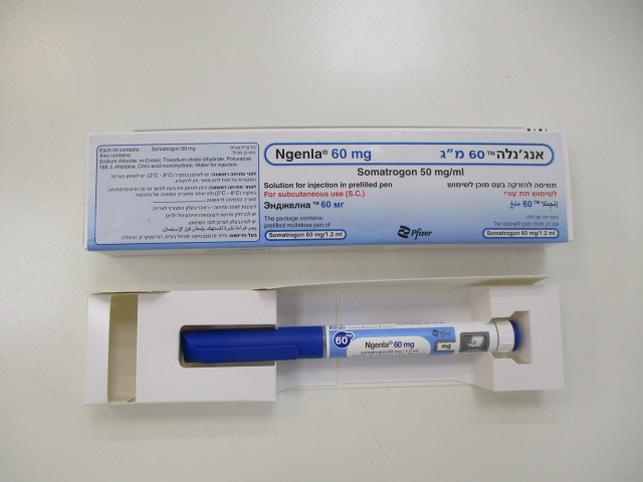
אנג'נלה 60 מ"ג NGENLA 60 MG (SOMATROGON)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile The commonly reported adverse reactions after treatment with somatrogon are injection site reactions (ISRs) (25.1%), headache (10.7% ) and pyrexia (10.2%). Tabulated list of adverse reactions Safety data are derived from the phase 2, multi-centre safety and dose-finding study, and the pivotal phase 3, multi-centre non-inferiority study in paediatric patients with GHD (see section 5.1). The data reflect exposure of 265 patients to somatrogon administered once weekly (0.66 mg/kg/week). Table 1 presents the adverse reactions for somatrogon within the system organ class (SOC). The adverse reactions listed in the table below are presented by SOC and frequency categories, defined using the following convention: very common (≥ 1/10); common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥ 1/10,000 to < 1/1,000), very rare (< 1/10,000) or frequency not known (cannot be estimated from the available data). Within each frequency grouping, adverse reactions are presented in the order of decreasing seriousness. Table 1. Adverse reactions System organ class Very Common Uncommon Rare Very Frequency common rare not known Blood and lymphatic Anaemia system disorders Eosinophilia Endocrine disorders Hypothyroidism Adrenal insufficiency Nervous system Headache disorders Eye disorders Conjunctivitis allergic Skin and subcutaneous Rash tissue disorders generalised Musculoskeletal and Arthralgia connective tissue Pain in extremity disorders General disorders and Injection site administration site reactionsa conditions Pyrexia System organ class Very Common Uncommon Rare Very Frequency common rare not known a Injection site reactions include the following: injection site pain, erythema, pruritus, swelling, induration, bruising, haemorrhage, warmth, hypertrophy, inflammation, deformation, urticaria. Description of selected adverse reactions Injection site reaction In the phase 3 clinical study, reporting of ISRs was actively solicited during the course of the study. In the majority of cases, local ISRs tended to be transient, occurred mainly in the first 6 months of treatment and were mild in severity; ISRs had a mean onset on the day of the injection and a mean duration of < 1 day. Among them, injection site pain, erythema, pruritus, swelling, induration, bruising, hypertrophy, inflammation and warmth were reported in 43.1% of patients treated with somatrogon compared to 25.2% of patients administered daily injections of somatropin. In the long-term OLE of the clinical phase 3 study, local ISRs were similar in nature and severity, and reported early in subjects switching from somatropin to somatrogon treatment. ISRs were reported in 18.3% of patients originally treated with somatrogon in the main study and continuing treatment in the OLE portion of the study, and likewise, 37% were reported among patients originally treated with somatropin that were switched in the OLE portion of the study to treatment with somatrogon. Immunogenicity In the pivotal safety and efficacy study, among 109 subjects treated with somatrogon, 84 (77.1%) tested positive for anti-drug antibodies (ADAs). There were no clinical or safety effects observed with the formation of antibodies. Other adverse drug reactions for somatropin may be considered class effects, such as: • Neoplasms benign and malignant: (see section 4.4). • Metabolism and nutrition disorders: diabetes mellitus type 2 (see section 4.4). • Nervous system disorders: benign intracranial hypertension (see section 4.4), paraesthesia. • Musculoskeletal, connective tissue, and bone disorders: myalgia. • Reproductive system and breast disorders: gynaecomastia. • Skin and subcutaneous tissue disorders: skin rash, urticaria and pruritus. • General disorders and administration site conditions: peripheral oedema, facial oedema. • Gastrointestinal disorders: pancreatitis (see section 4.4). Metacresol This medicinal product contains metacresol which may contribute to painful injections (see section 4.4). Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
