Quest for the right Drug
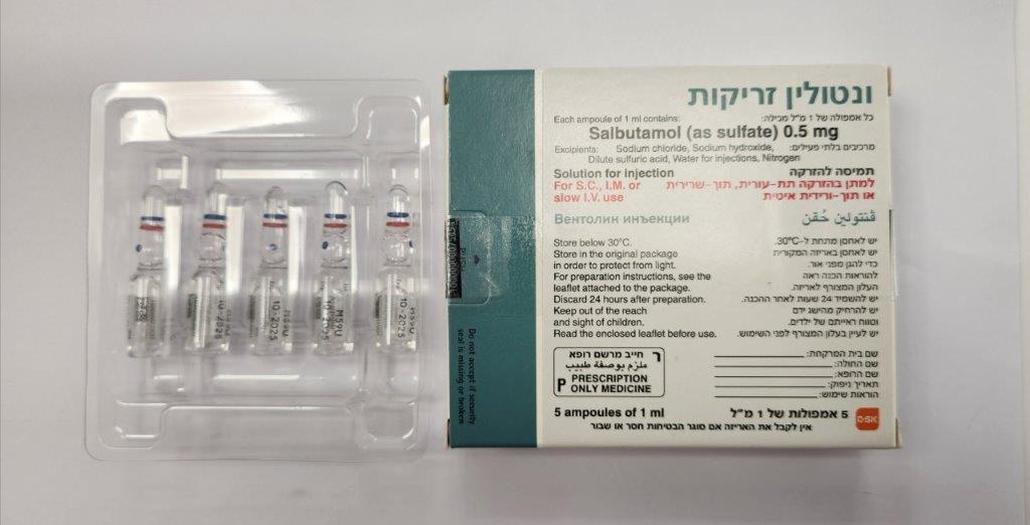
ונטולין זריקות VENTOLIN INJECTION (SALBUTAMOL AS SULFATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי, תת-עורי : I.M, I.V, S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8. Undesirable Effects Adverse events are listed below by system organ class and frequency. Frequencies are defined as: very common (1/10), common (1/100 and 1/10), uncommon (1/1000 and 1/100), rare (1/10,000 and 1/1000) and very rare (1/10,000). Very common and common events were generally determined from clinical trial data. Rare, very rare and unknown events were generally determined from spontaneous data. Immune system disorders Very rare: Hypersensitivity reactions including angioedema, urticaria, bronchospasm, hypotension and collapse. Metabolism and nutrition disorders Rare: Hypokalaemia. Potentially serious hypokalaemia may result from beta-agonist therapy. Unknown: Lactic acidosis (see section 4.4) Nervous system disorders Very common: Tremor. Common: Headache. Very rare: Hyperactivity. Cardiac disorders Very common: Tachycardia. Common: Palpitations. Rare: Cardiac arrhythmias including atrial fibrillation, supraventricular tachycardia and extrasystoles. Unknown: Myocardial ischaemia* ( see section 4.4) Vascular disorders Rare: Peripheral vasodilatation. Gastrointestinal disorders Unknown: Nausea, vomiting *. Musculoskeletal and connective tissue disorders Common: Muscle cramps. Injury, poisoning and procedural complications Unknown: Slight pain or stinging on intramuscular use of undiluted injection*. * reported spontaneously in post-marketing data therefore frequency regarded as unknown. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il Additionally, you should also report to GSK Israel (il.safety@gsk.com).

שימוש לפי פנקס קופ''ח כללית 1994
Bronchial asthma, bronchospasm
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
