Quest for the right Drug
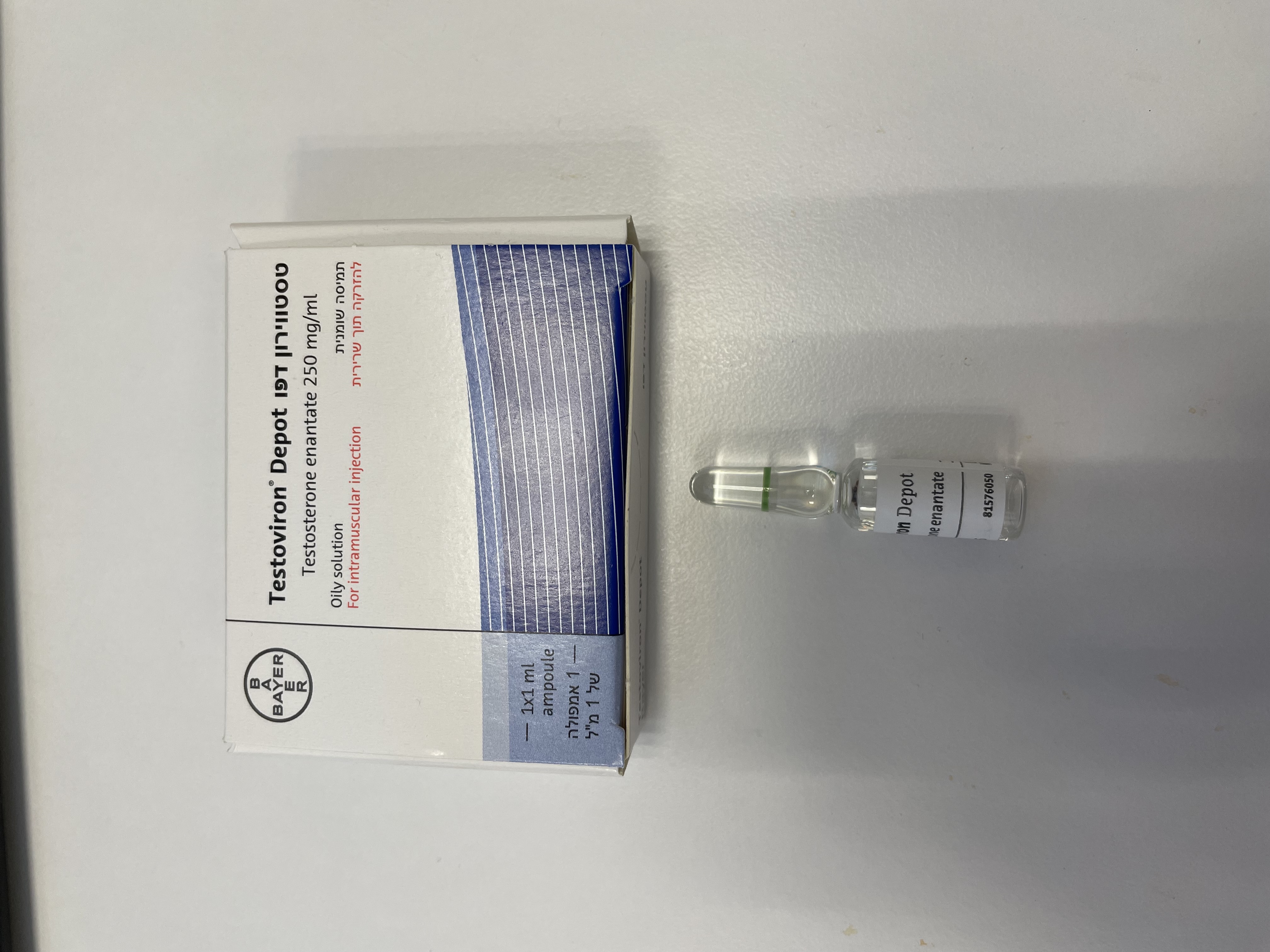
טסטווירון דפו TESTOVIRON DEPOT (TESTOSTERONE ENANTATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
תמיסה שומנית להזרקה : OILY SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Elderly patients treated with androgens may be at increased risk of developing prostatic hyperplasia. There is no clear evidence that androgens actually cause prostate cancer, but androgens can potentiate the growth of existing prostate cancer. Existing prostate carcinoma should therefore be excluded before use of testosterone preparations. For the treatment of hypogonadism, Testoviron Depot may be used only if hypogonadism (hyper or hypogonadotropic) has been demonstrated and if other aetiology, responsible for the symptoms, has been excluded. Testosterone insufficiency must be clearly demonstrated in the clinical symptoms (regression of secondary sexual characteristics, change in body composition, asthenia, reduced libido, erectile dysfunction, etc.) and confirmed by two separate blood testosterone measurements. There is limited experience on the safety and efficacy of the use of Testoviron Depot in patients over 65 years of age. Currently, there is no consensus about age-specific testosterone reference values. However, it should be taken into account that physiologically testosterone serum levels are lower with increasing age. In children, testosterone may accelerate bone maturation as a result of peripheral conversion to oestrogen, thereby reducing adult height. In longer-term or higher-dose administration, radiological bone age measurements should therefore be conducted at regular intervals. Testoviron Depot must not be used in women, as women may develop signs of virilisation, e.g. acne, hirsutism, voice changes (particular care is required in women professionally reliant on singing or speaking), depending on individual sensitivity to androgenic impulses. Testoviron Depot is not suitable for the treatment of male sterility. Venous Thromboembolism There have been postmarketing reports of venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), in patients using testosterone products, such as Testoviron Depot. Evaluate patients who report symptoms of pain, edema, warmth and erythema in the lower extremity for DVT and those who present with acute shortness of breath for PE. If a venous thromboembolic event is suspected, discontinue treatment with Testoviron Depot and initiate appropriate workup and management. Medical examination Before the start of therapy with testosterone, all patients must undergo a detailed medical examination in order to exclude the risk of pre-existing prostatic cancer. In patients receiving testosterone therapy, careful and regular check-ups of the prostate gland and breast must be performed in accordance with currently established methods (digital rectal examination and monitoring of serum PSA) at least once yearly, or twice yearly in elderly patients and in patients at risk (with certain clinical or familial factors). Testosterone level should be monitored at baseline and at regular intervals during treatment. Clinicians should adjust the dosage individually to ensure maintenance of eugonadal testosterone levels. In patients receiving long-term androgen therapy the following laboratory parameters should also be monitored regularly: haemoglobin, and haematocrit, liver function tests and lipid profile (see section 4.8). Due to variability in laboratory values, all measuring of testosterone levels should be carried out in the same laboratory. Tumours Androgens may accelerate the development of sub-clinical prostatic cancer and benign prostatic hyperplasia. Testoviron Depot should be used with caution in cancer patients at risk of hypercalcaemia (and associated hypercalciuria), e.g due to bone metastasis; see also section 4.3. It is recommended that serum calcium levels be regularly monitored in these patients. Cases of benign and malignant liver tumours that can lead to life-threatening intra-abdominal bleeding have been observed following use of testosterone depot preparations. Blood clotting disorders As a general rule, the limitations of using intramuscular injections in patients with acquired or inherited bleeding disorders must always be observed. Testosterone and its derivatives have been reported to increase the effect of coumarin-derived anticoagulants (see section 4.5). Testosterone should be used with caution in patients with thrombophilia or risk factors for venous thromboembolism (VTE), as there have been post-marketing studies and reports of thrombotic events (e.g. deep-vein thrombosis, pulmonary embolism, ocular thrombosis) in these patients during testosterone therapy. In thrombophilic patients, VTE cases have been reported even under anticoagulation treatment, therefore continuing testosterone treatment after first thrombotic event should be carefully evaluated. In case of treatment continuation, further measures should be taken to minimise the individual VTE risk. Other diseases In patients suffering from severe cardiac, hepatic or renal insufficiency or ischaemic heart disease, treatment with testosterone can cause severe complications, characterised by oedema, with or without congestive cardiac failure. In this case, treatment must be stopped immediately. Caution should be exercised in patients predisposed to oedema, as treatment with androgens can exacerbate sodium retention (see section 4.8). Studies on the efficacy and safety of this medicinal product have not been conducted in patients with impaired renal or hepatic function. Testosterone therapy must therefore be performed only with caution in these patients. Testosterone may cause a rise in blood pressure and Testoviron Depot should be used in caution in men with hypertension. Testoviron Depot should be used only with caution in patients with epilepsy or migraine, as it may aggravate these disorders. In diabetic patients treated with androgens who achieve normal plasma testosterone levels after testosterone therapy, there may be a reduction in blood glucose, and hence a decrease in the need for insulin. Certain clinical symptoms, such as irritability, nervousness, weight gain, persistent or frequent erections may indicate excessive androgen exposure and require a dose adjustment (see also section 4.2). Testoviron Depot should be permanently discontinued if symptoms of excessive androgen exposure persist or recur during therapy on the recommended dosing schedule. Pre-existing sleep apnoea may be exacerbated. Drug abuse and dependence Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication(s) and in combination with other anabolic androgenic steroids. Abuse of testosterone and other anabolic androgenic steroids can lead to serious adverse reactions including: cardiovascular (with fatal outcomes in some cases), hepatic and/or psychiatric events. Testosterone abuse may result in dependence and withdrawal symptoms upon significant dose reduction or abrupt discontinuation of use. The abuse of testosterone and other anabolic androgenic steroids carries serious health risks and is to be discouraged. Administration Like all oily solutions, Testoviron Depot must be injected precisely and very slowly via the intramuscular route. A pulmonary microembolism with oily solutions can lead to symptoms such as cough, dyspnoea, malaise, hyperhidrosis, chest pain, dizziness, paraesthesia or syncope. These reactions can occur during or immediately after the injection and are reversible. Treatment is usually carried out with supportive measures, e.g. with additional oxygen administration. Excipient information This medicinal product contains 342.0 mg benzyl benzoate in each ampoule/pre-filled syringe. The use of Testoviron Depot can lead to positive results in doping tests. Androgens such as those contained in Testoviron Depot are not suitable for enhancing muscular development in healthy individuals or for boosting physical performance. It is impossible to predict the health consequences of using Testoviron Depot as a doping agent; serious health risks cannot be ruled out (see section 4.8).
Effects on Driving
4.7 Effects on ability to drive and use machines Testoviron Depot has no or negligible influence on the ability to drive and use machines.

שימוש לפי פנקס קופ''ח כללית 1994
Androgen deficiency states in men, breast cancer in women, aplastic anemia
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
