Quest for the right Drug
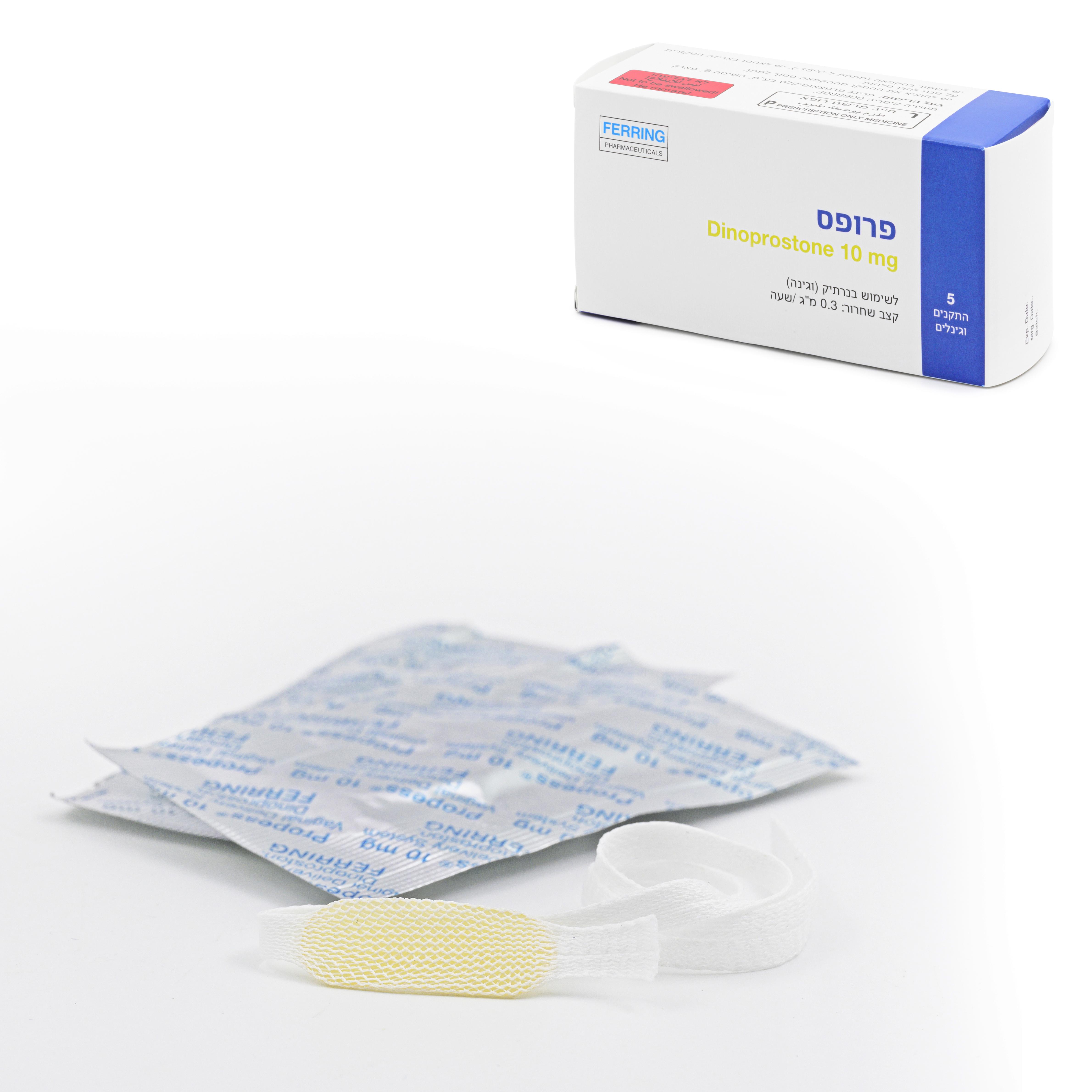
פרופס PROPESS (DINOPROSTONE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
וגינלי : VAGINAL
צורת מינון:
התקן תוך נרתיקי : PESSARY
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of safety profile: The most commonly reported adverse drug reactions in placebo-controlled and active comparator efficacy clinical trials (N=1116) were “foetal heart rate disorder” (6.9%), “uterine contractions abnormal” (6.2%) and “abnormal labour affecting foetus” (2.6 %). The table below displays the main ADRs distributed by system organ classes (SOC) and frequency. Further, the ADRs seen during post-marketing experience are mentioned with unknown frequency. Adverse reactions observed in clinical studies are presented according to their incidence, post authorisation reported adverse reactions are presented in the column frequency unknown. System organ class Common Uncommon Not known: frequency (≥ 1/100 and < 1/10) (≥ 1/1000 and ≤ 1/100) cannot be estimated from the available data Blood and lymphatic Disseminated system disorders intravascular coagulation Immune system Anaphylactic reaction disorders Hypersensitivity Nervous system Headache disorders Cardiac disorders Foetal heart rate disorder 1* Vascular disorders Hypotension Respiratory, thoracic Neonatal respiratory and mediastinal distress related disorders conditions Gastrointestinal Abdominal pain, disorders Nausea, vomiting, diarrhoea Hepatobiliary disorders Neonatal hyperbilirubinaemia Skin and subcutaneous Pruritus tissue disorders Pregnancy, puerperium Abnormal labour Postpartum Anaphylactoid and perinatal conditions affecting foetus 2* haemorrhage, syndrome of pregnancy Uterine contractions Premature separation of Foetal distress abnormal, uterine placenta, syndrome 3* tachysystole, uterine Apgar score low Fetal death, stillbirth, hyperstimulation, neonatal death 4* Arrested labour uterine hypertonus Chorioamnionitis Meconium in amniotic fluid Uterine atony Reproductive system Vulvovaginal burning Genital oedema and breast disorders sensation General disorders and Febrile disorders administration site conditions Injury, poisoning and Uterine rupture procedural complications 1* “Foetal heart rate disorder” was in clinical studies reported as “foetal heart rate abnormalities”, “foetal bradycardia”, “foetal tachycardia”, “unexplained absence of normal variability”, “foetal heart rate decreased”, “foetal heart rate deceleration”, “early or late decelerations”, “variable decelerations”, “prolonged decelerations”. 2* “Abnormal labour affecting foetus” as expression for hyperstimulation syndrome was in clinical studies reported as “uterine tachysystole” combined with “late decelerations”, “foetal bradycardia”, or “prolonged decelerations” 3* “Foetal distress syndrome” was also reported as “foetal acidosis” , “pathological CTG”, “foetal heart rate abnormalities”, “intrauterine hypoxia” or “threatening asphyxia”. The term itself is unspecific, has a low positive predictive value and is often associated with an infant who is in good condition at birth. 4* Fetal death, stillbirth, and neonatal death have been reported after application of dinoprostone, especially following the occurrence of serious events such as uterine rupture (see sections 4.2, 4.3 and 4.4). Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/2000
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
