Quest for the right Drug
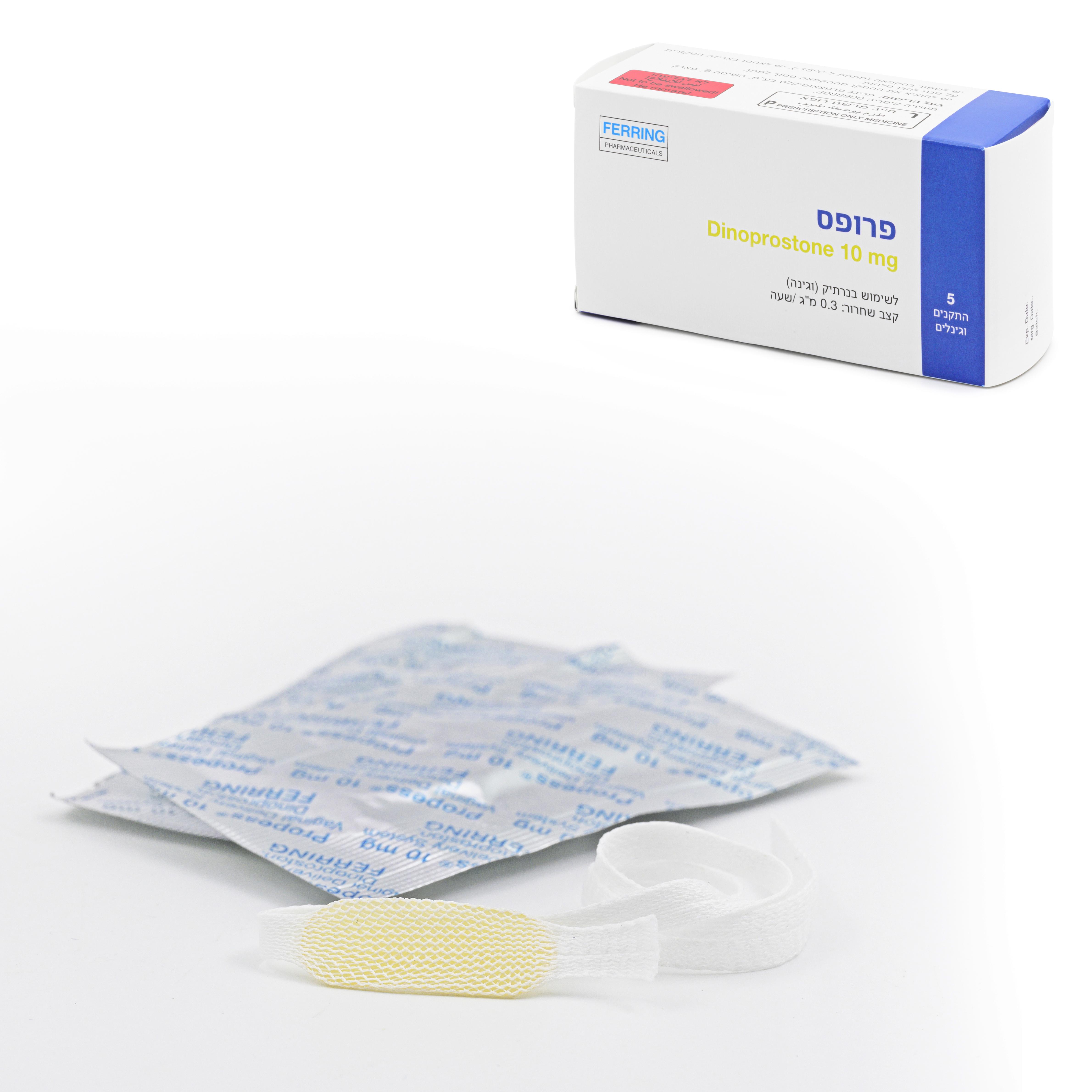
פרופס PROPESS (DINOPROSTONE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
וגינלי : VAGINAL
צורת מינון:
התקן תוך נרתיקי : PESSARY
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: uterotonics, prostaglandins ATC-code: G02AD02 Prostaglandin E2 (PGE2) is a naturally occurring compound found in low concentrations in most tissues of the body. It functions as a local hormone. Prostaglandin E2 plays an important role in the complex set of biochemical and structural alterations involved in cervical ripening. Cervical ripening involves a transformation of the uterine cervix which must be transformed from a rigid structure to a soft, dilated configuration to allow passage of the fetus through the birth canal. This process involves activation of the enzyme collagenase which is responsible for the breakdown of the collagen. Local administration of dinoprostone to the cervix results in cervical ripening which then induces the subsequent events which complete labour.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties PGE2 is rapidly metabolised primarily in the tissue of synthesis. Any which escapes local inactivation is rapidly cleared from the circulation with a half-life generally estimated as 1-3 minutes. No correlation could be established between PGE2 release and plasma concentrations of its metabolite, PGEm. The relative contributions of endogenously and exogenously released PGE2 to the plasma levels of the metabolite PGEm could not be determined. The reservoir of 10 mg dinoprostone serves to maintain a controlled and constant release. The release rate is approximately 0.3 mg per hour over 24 hours in women with intact membranes whereas release is higher and more variable in women with premature rupture of membranes. PROPESS releases dinoprostone to the cervical tissue continuously at a rate which allows cervical ripening to progress until complete, and with the facility to remove the dinoprostone source when the clinician decides that cervical ripening is complete or labour has started, at which point no further dinoprostone is required.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/2000
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
