Quest for the right Drug
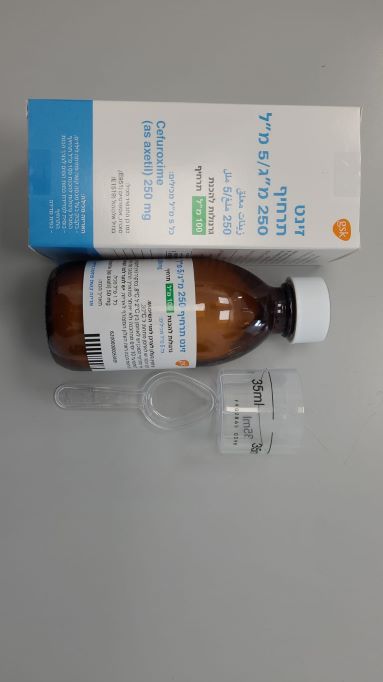
זינט תרחיף 250 מ"ג/5 מ"ל ZINNAT SUSPENSION 250 MG/5 ML (CEFUROXIME AS AXETIL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
אין פרטים : GRANULES FOR SUSPENSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: antibacterials for systemic use, second-generation cephalosporins, ATC code: J01DC02 Mechanism of action Cefuroxime axetil undergoes hydrolysis by esterase enzymes to the active antibiotic, cefuroxime. Cefuroxime inhibits bacterial cell wall synthesis following attachment to penicillin binding proteins (PBPs). This results in the interruption of cell wall (peptidoglycan) biosynthesis, which leads to bacterial cell lysis and death. Mechanism of resistance Bacterial resistance to cefuroxime may be due to one or more of the following mechanisms: • hydrolysis by beta-lactamases; including (but not limited to) by extended-spectrum beta-lactamases (ESBLs), and AmpC enzymes that may be induced or stably derepressed in certain aerobic Gram-negative bacteria species; • reduced affinity of penicillin-binding proteins for cefuroxime; • outer membrane impermeability, which restricts access of cefuroxime to penicillin binding proteins in Gram- negative bacteria; • bacterial efflux pumps. Organisms that have acquired resistance to other injectable cephalosporins are expected to be resistant to cefuroxime. Depending on the mechanism of resistance, organisms with acquired resistance to penicillins may demonstrate reduced susceptibility or resistance to cefuroxime. Cefuroxime axetil breakpoints Minimum inhibitory concentration (MIC) breakpoints established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) are as follows: Microorganism Breakpoints (mg/L) S R Enterobacteriaceae1, 2 ≤8 >8 Staphylococcus spp. Note3 Note3 Streptococcus A, B, C and G Note4 Note4 Streptococcus pneumoniae ≤0.25 >0.5 Moraxella catarrhalis ≤0.125 >4 Haemophilus ≤0.125 >1 influenzae Non-species related IE5 IE5 breakpoints1 1 The cephalosporin breakpoints for Enterobacteriaceae will detect all clinically important resistance mechanisms (including ESBL and plasmid mediated AmpC). Some strains that produce beta-lactamases are susceptible or intermediate to 3rd or 4th generation cephalosporins with these breakpoints and should be reported as found, i.e. the presence or absence of an ESBL does not in itself influence the categorization of susceptibility. In many areas, ESBL detection and characterization is recommended or mandatory for infection control purposes. 2 Uncomplicated UTI (cystitis) only (see section 4.1). 3 Susceptibility of staphylococci to cephalosporins is inferred from the methicillin susceptibility except for ceftazidme and cefixime and ceftibuten, which do not have breakpoints and should not be used for staphylococcal infections. 4 The beta-lactam susceptability of beta-haemolytic streptococci groups A, B, C and G is inferred from the penicillin susceptibility. 5 insufficient evidence that the species in question is a good target for therapy with the drug. An MIC with a comment but without an accompanying S or R-categorization may be reported. S=susceptible, R=resistant Microbiological susceptibility The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of cefuroxime axetil in at least some types of infections is questionable. Cefuroxime is usually active against the following microorganisms in vitro. Commonly susceptible species Gram-positive aerobes: Staphylococcus aureus (methicillin susceptible)* Coagulase negative staphylococcus (methicillin susceptible) Streptococcus pyogenes Streptococcus agalactiae Gram-negative aerobes: Haemophilus influenzae Haemophilus parainfluenzae Moraxella catarrhalis Spirochaetes: Borrelia burgdorferi Microorganisms for which acquired resistance may be a problem Gram-positive aerobes: Streptococcus pneumoniae Gram-negative aerobes: Citrobacter freundii Enterobacter aerogenes Enterobacter cloacae Escherichia coli Klebsiella pneumoniae Proteus mirabilis Proteus spp.(other than P. vulgaris) Providencia spp. Gram-positive anaerobes: Peptostreptococcus spp. Propionibacterium spp. Gram-negative anaerobes: Fusobacterium spp. Bacteroides spp. Inherently resistant microorganisms Gram-positive aerobes: Enterococcus faecalis Enterococcus faecium Gram-negative aerobes: Acinetobacter spp. Campylobacter spp. Morganella morganii Proteus vulgaris Pseudomonas aeruginosa Serratia marcescens Gram-negative anaerobes: Bacteroides fragilis Others: Chlamydia spp. Mycoplasma spp. Legionella spp. *All methicillin-resistant S. aureus are resistant to cefuroxime.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Absorption After oral administration cefuroxime axetil is absorbed from the gastrointestinal tract and rapidly hydrolysed in the intestinal mucosa and blood to release cefuroxime into the circulation. Optimum absorption occurs when it is administered shortly after a meal. Following administration of cefuroxime axetil tablets peak serum levels (2.1 mcg/ml for a 125 mg dose, 4.1 mcg/ml for a 250 mg dose, 7.0 mcg/ml for a 500 mg dose and 13.6 mcg/ml for a 1000 mg dose) occur approximately 2 to 3 hours after dosing when taken with food. The rate of absorption of cefuroxime from the suspension is reduced compared with the tablets, leading to later, lower peak serum levels and reduced systemic bioavailability (4 to 17% less). Cefuroxime axetil oral suspension was not bioequivalent to cefuroxime axetil tablets when tested in healthy adults and therefore is not substitutable on a milligram-per-milligram basis (see section 4.2).The pharmacokinetics of cefuroxime is linear over the oral dosage range of 125 to 1000 mg. No accumulation of cefuroxime occurred following repeat oral doses of 250 to 500 mg. Distribution Protein binding has been stated as 33 to 50% depending on the methodology used. Following a single dose of cefuroxime axetil 500 mg tablet to 12 healthy volunteers, the apparent volume of distribution was 50 L (CV%=28%). Concentrations of cefuroxime in excess of the minimum inhibitory levels for common pathogens can be achieved in the tonsilla, sinus tissues, bronchial mucosa, bone, pleural fluid, joint fluid, synovial fluid, interstitial fluid, bile, sputum and aqueous humor. Cefuroxime passes the blood-brain barrier when the meninges are inflamed. Biotransformation Cefuroxime is not metabolised. Elimination The serum half-life is between 1 and 1.5 hours. Cefuroxime is excreted by glomerular filtration and tubular secretion. The renal clearance is in the region of 125 to 148 ml/min/1.73 m2. Special patient populations Gender No differences in the pharmacokinetics of cefuroxime were observed between males and females. Elderly No special precaution is necessary in the elderly patients with normal renal function at dosages up to the normal maximum of 1 g per day. Elderly patients are more likely to have decreased renal function; therefore, the dose should be adjusted in accordance with the renal function in the elderly (see section 4.2). Paediatrics In older infants (aged >3 months) and in children, the pharmacokinetics of cefuroxime are similar to that observed in adults. There is no clinical trial data available on the use of cefuroxime axetil in children under the age of 3 months. Renal impairment The safety and efficacy of cefuroxime axetil in patients with renal failure have not been established. Cefuroxime is primarily excreted by the kidneys. Therefore, as with all such antibiotics, in patients with markedly impaired renal function (i.e. C1cr <30 ml/minute) it is recommended that the dosage of cefuroxime should be reduced to compensate for its slower excretion (see section 4.2). Cefuroxime is effectively removed by dialysis. Hepatic impairment There are no data available for patients with hepatic impairment. Since cefuroxime is primarily eliminated by the kidney, the presence of hepatic dysfunction is expected to have no effect on the pharmacokinetics of cefuroxime. Pharmacokinetic/pharmacodynamic relationship For cephalosporins, the most important pharmacokinetic-pharmacodynamic index correlating with in vivo efficacy has been shown to be the percentage of the dosing interval (%T) that the unbound concentration remains above the minimum inhibitory concentration (MIC) of cefuroxime for individual target species (i.e. %T>MIC).

שימוש לפי פנקס קופ''ח כללית 1994
Upper & lower respiratory tract infections (sinusitis, otitis, chronic bronchitis, pneumonia) genitourinary infections, pyelonephritis caused by: staphylococcus aureus & epidermidis (excluding methicillin resistant strains), streptococci (excluding enterococci), H. influenzae (including beta lactamase resistant strains) Branhamella catarrhalis, E. coli, klebsiella species, proteus mirabilis, proteus rettgeri, providencia, N. gonorrhea (including penicillinase producing strains)
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
