Quest for the right Drug
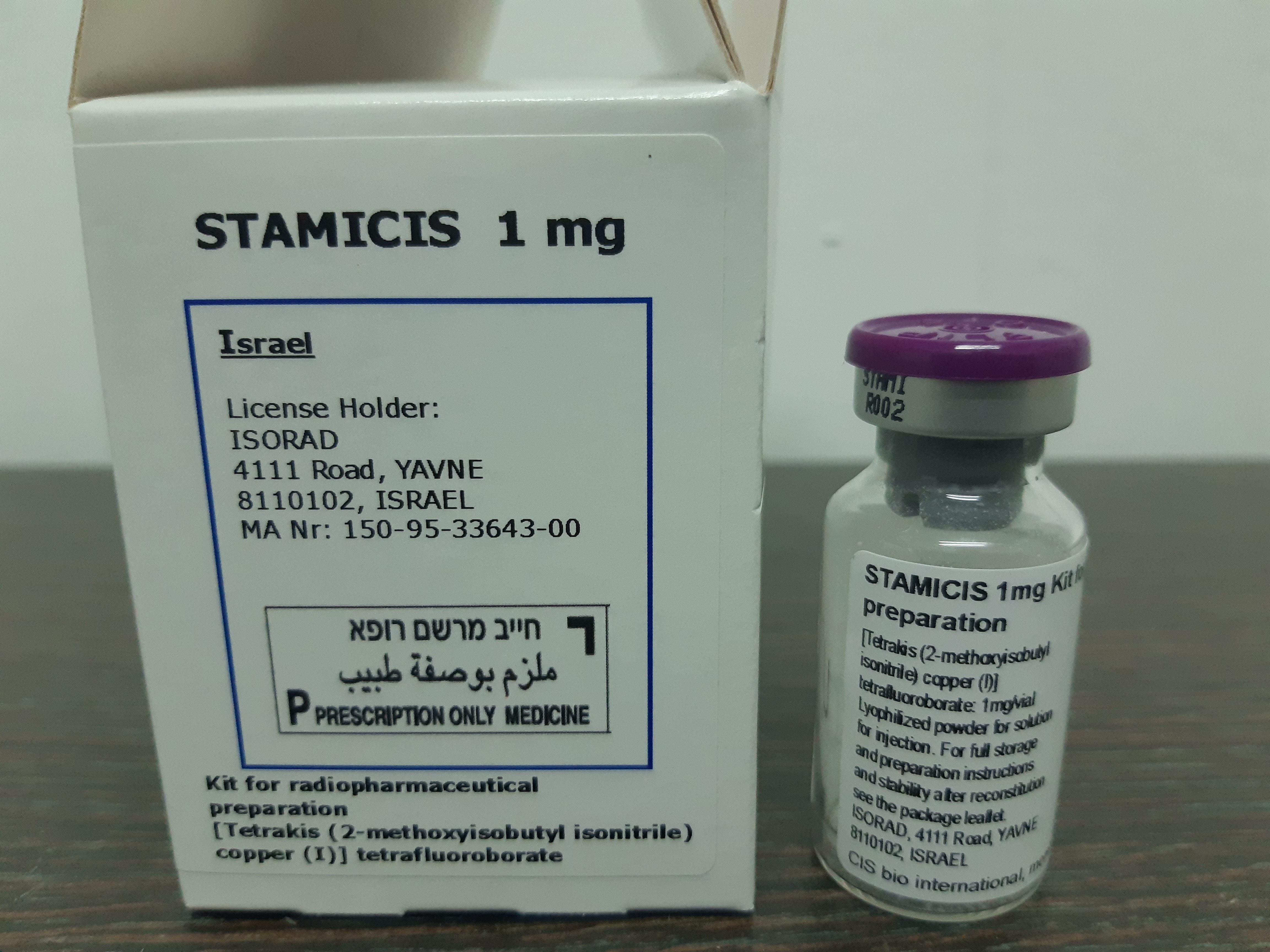
סטמיציס STAMICIS 1 MG KIT FOR RADIOPHARMACEUTICAL PREPATION (TETRAKIS COPPER (I) TETRAFLUOROBORATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אבקה מיובשת בהקפאה להכנת תמיסה להזרקה : LYOPHILIZED POWDER FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The following table presents how the frequencies are reflected in this section: Very common (1/10) Common (1/100 to <1/10) Uncommon (1/1,000 to <1/100) Rare (1/10,000 to <1/1,000) Very rare (<1/10,000) Not known (cannot be estimated from the available data) Immune system disorders: Rare: Severe hypersensitivity reactions such as dyspnoea, hypotension, bradycardia, asthenia and vomiting (usually within two hours of administration), angioedema. Other hypersensitivity reactions (allergic skin and mucosa reactions with exanthema (pruritus, urticaria, oedema), vasodilatation). Very rare: Other hypersensitivity reactions have been described in predisposed patients. Nervous system disorders: Uncommon: Headache. Rare: Seizures (shortly after administration), syncope. Cardiac disorders Uncommon: Chest pain/angina pectoris, abnormal ECG. Rare: Arrhythmia. Gastrointestinal disorders: Uncommon: Nausea. Rare: Abdominal pain. Skin and subcutaneous tissue disorders: Rare: local reactions at the injection site, hypoaesthesia and paraesthesia, flushing. Not known: Erythema multiforme. General disorders and administration site conditions: Common: Immediately after injection, a metallic or bitter taste, partly in combination with dry mouth and an alteration in the sense of smell may be observed. Rare: Fever, fatigue, dizziness, transient arthritic-like pain. Other disorders Exposure to ionising radiation is linked with cancer induction and a potential for development of hereditary defects. As the effective dose is 16.4 mSv when the maximal recommended activity of 2000 MBq (500 at rest and 1500 MBq at stress) for a 1-day-protocol is administered these adverse reactions are expected to occur with a low probability. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
ATC
מידע נוסף
