Quest for the right Drug
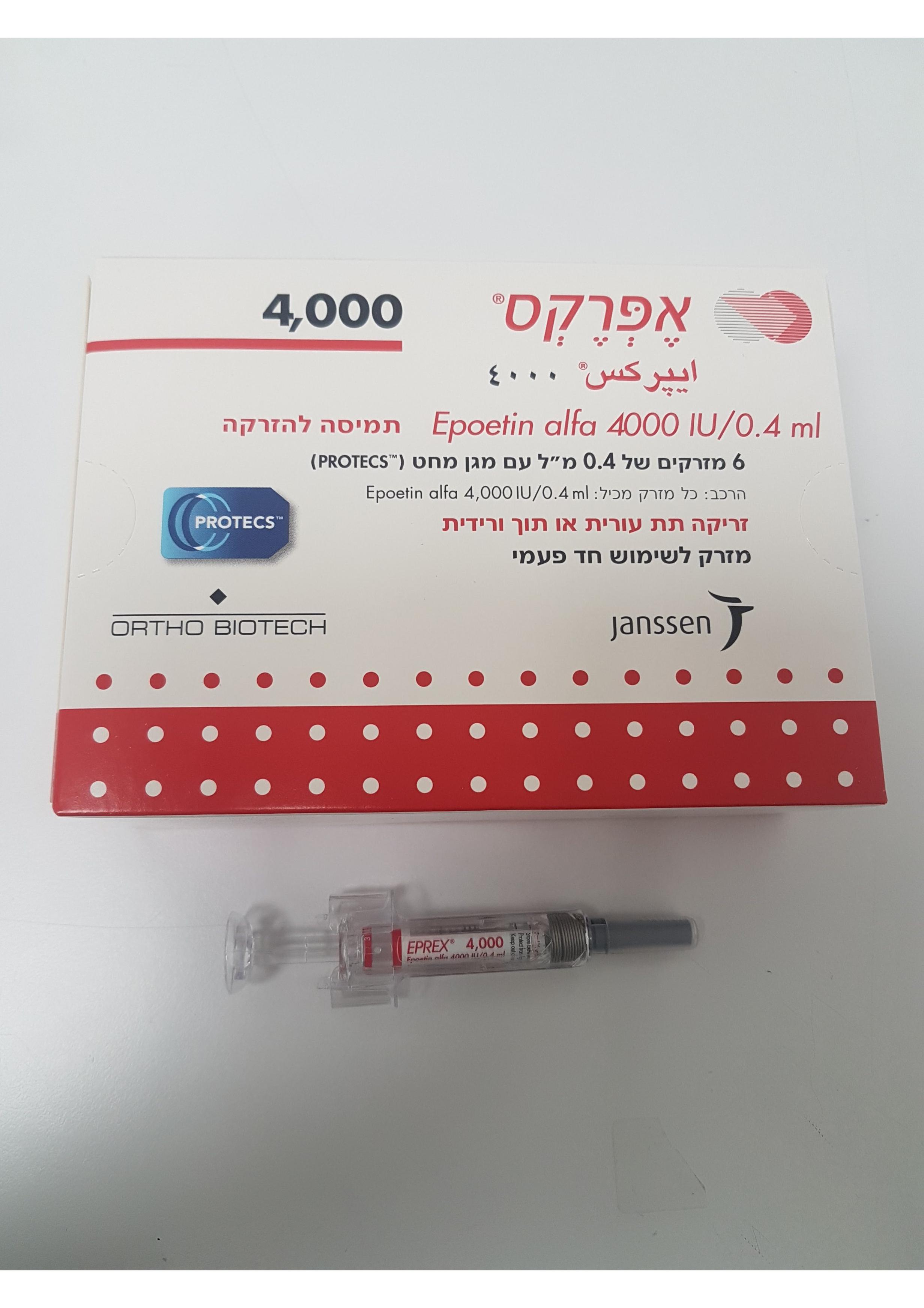
אפרקס 4000 EPREX 4000 (RECOMBINANT HUMAN ERYTHROPOIETIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי, תת-עורי : I.V, S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6 PHARMACEUTICAL PARTICULARS 6.1 List of excipients Glycine Sodium chloride Disodium phosphate dihydrate Sodium dihydrogen phosphate dihydrate Polysorbate 80 Water for injections 6.2 Incompatibilities In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. 6.4 Special precautions for storage Store in a refrigerator (2°C to 8°C). This temperature range should be closely maintained until administration to the patient. Store in the original package in order to protect from light. Do not freeze or shake. For the purpose of ambulatory use, the product may be taken out of the refrigerator, without being replaced, for a maximum period of 3 days at a temperature not above 25°C. If the medicine has not been used at the end of this period, it should be disposed of. 6.5 Nature and contents of container EPREX 2,000 IU/mL solution for injection in pre-filled syringe 0.5 mL (1,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack size of 6. EPREX 4,000 IU/mL solution for injection in pre-filled syringe 0.5 mL (2,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of Eprex_PI_Sep2021_Ref_EU SmPC Jun2021 Page 24 of 25 latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack size of 6. EPREX 10,000 IU/mL solution for injection in pre-filled syringe 0.3 mL (3,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack size of 6. 0.4 mL (4,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack size of 6. 0.5 mL (5,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle caver (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack size of 6. 0.6 mL (6,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack size of 6. 0.8 mL (8,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack size of 6. 1.0 mL (10,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack size of 6 EPREX 40,000 IU/mL solution for injection in pre-filled syringe 0.5 mL (20,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack sizes of 1,4 or 6. 0.75 mL (30,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon-faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack sizes of 1,4 or 6. 1.0 mL (40,000 IU) of solution for injection in a pre-filled syringe (type I glass) with plunger (Teflon- faced rubber) and needle with a needle cover (liner contains dry natural rubber [a derivative of latex] with polypropylene cap) and a PROTECS™ needle guard device (polycarbonate) attached to the syringe - pack sizes of 1,4 or 6. 6.6 Special precautions for disposal and other handling The product should not be used, and discarded • if the seal is broken, • if the liquid is coloured or you can see particles floating in it, Eprex_PI_Sep2021_Ref_EU SmPC Jun2021 Page 25 of 25 • if you know, or think that it may have been accidentally frozen, or • if there has been a refrigerator failure. The product is for single use only. Only take one dose of EPREX from each syringe. In case only a partial dose of the syringe is required, the cover should be removed before the plunger is pushed up to the desired graduation mark to remove unwanted solution before injection. Refer to section 3. How to use EPREX (instructions on how to inject EPREX) of the package leaflet. The pre-filled syringes are fitted with the PROTECS™ needle guard device to help prevent needle stick injuries after use. The package leaflet includes full instructions for the use and handling of pre-filled syringes with the PROTECS™ needle guard. Any unused medicinal product or waste material should be disposed of in accordance with local requirements. 6.7 Graduation marks The syringe label contains numbered graduation marks to provide for the administration of a part of the dose (see Section 6.6). However, the product is for single use only. Only one dose of EPREX from each syringe should be taken 7 MARKETING AUTHORISATION HOLDER J-C Health Care Ltd., Kibbutz Shefayim 6099000, Israel.

פרטי מסגרת הכללה בסל
התרופה תינתן בכל אחד מאלה: 1. אנמיה חמורה (severe anemia) בחולי אי ספיקה כלייתית כרונית. 2. חולים אנמיים הסובלים ממחלה ממאירה והמקבלים טיפול פעיל ייעודי במחלתם וכן לחולים הסובלים ממיאלומה נפוצה (multiple myeloma) או מהתסמונת המיאלודיספלסטית (myelodisplastic syndrome) שנתקיימו בהם כל אלה: 1. אחד מהתנאים האלה: א. רמת המוגלובין נמוכה מ-8 גרם %. ב. החולה מרותק למיטתו בגלל אנמיה המלווה במחלת לב איסכמית או באי ספיקה לבבית. ג. החולה נזקק לקבלת שתי מנות דם לפחות פעם בשבועיים במשך חודשיים. 2. נשללה סיבה אחרת לאנמיה שאינה קשורה לטיפול הייעודי במחלתם האמורה לעיל ובכלל זה דימום, חוסר ברזל, חוסר חומצה פולית, חוסר ויטמין B12 והמוליזה. 3. רמת אריתרופואטין בנסיוב נמוכה מ-100 mu/ml.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| חולים אנמיים הסובלים ממחלה ממאירה והמקבלים טיפול פעיל ייעודי במחלתם וכן לחולים הסובלים ממיאלומה נפוצה (multiple myeloma) או מהתסמונת המיאלודיספלסטית (myelodisplastic syndrome | 01/02/2001 | המטולוגיה | ||
| אנמיה חמורה (severe anemia) בחולי אי ספיקה כלייתית כרונית. | 01/02/2001 | המטולוגיה | ||
| oncology | 01/02/2001 | DARBEPOETIN ALFA, EPOETIN ALFA, EPOETIN BETA, EPOETIN THETA (R-HUEPO) | ||
| CKD | 01/02/2001 | DARBEPOETIN ALFA, EPOETIN BETA, EPOETIN ALFA, METHOXY POLYETHYLENE GLYCOL-EPOETIN BETA, EPOETIN THETA (R-HUEPO) |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/02/2001
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
