Quest for the right Drug
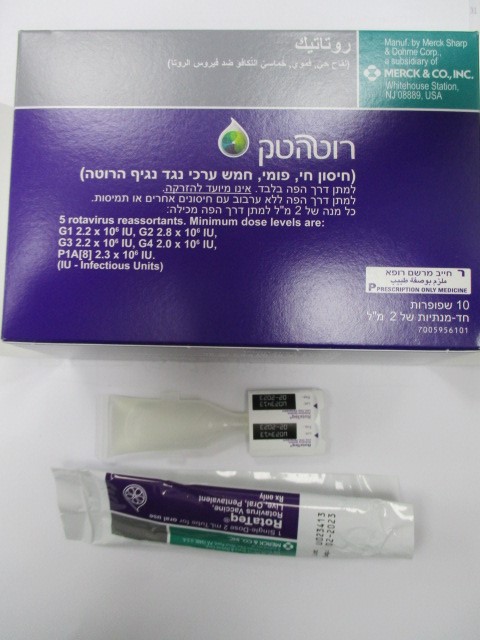
רוטטק ROTATEQ (ROTAVIRUS G1 REASSORTANT, ROTAVIRUS G2 REASSORTANT, ROTAVIRUS G3 REASSORTANT, ROTAVIRUS G4 REASSORTANT, ROTAVIRUS P1A[8] REASSORTANT)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
אין פרטים : ORAL SOLUTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Overdose : מינון יתר
12 OVERDOSAGE There have been post-marketing reports of infants who received more than one dose or a replacement dose of RotaTeq after regurgitation [see Dosage and Administration (5.2)]. In limited post-marketing experience of reported overdosage, the adverse events reported after incorrect administration of higher than recommended doses of RotaTeq were similar to adverse events observed with the approved dosage and schedule. 13 DESCRIPTION RotaTeq is a live, oral pentavalent vaccine that contains 5 live reassortant rotaviruses. The rotavirus parent strains of the reassortants were isolated from human and bovine hosts. Four reassortant rotaviruses express one of the outer capsid proteins (G1, G2, G3, or G4) from the human rotavirus parent strain and the attachment protein (type P7) from the bovine rotavirus parent strain. The fifth reassortant virus expresses the attachment protein, P1A (genotype P[8]), herein referred to as type P1A[8], from the human rotavirus parent strain and the outer capsid protein of type G6 from the bovine rotavirus parent strain (see Table 7). Table 7 Human Rotavirus Bovine Rotavirus Reassortant Outer Surface Parent Strains Parent Strain and Protein Composition Name of and Outer Surface Outer Surface Protein (Human Rotavirus Minimum Dose Levels Reassortant Protein Compositions Composition Component in Bold) (106 infectious units) G1 WI79 – G1P1A[8] G1P7[5] 2.2 G2 SC2 – G2P2[6] G2P7[5] 2.8 G3 WI78 – G3P1A[8] WC3 - G6, P7[5] G3P7[5] 2.2 G4 BrB – G4P2[6] G4P7[5] 2.0 P1A[8] WI79 – G1P1A[8] G6P1A[8] 2.3 The reassortants are propagated in Vero cells using standard cell culture techniques in the absence of antifungal agents. The reassortants are suspended in a buffered stabilizer solution. Each vaccine dose contains sucrose, sodium citrate, sodium phosphate monobasic monohydrate, sodium hydroxide, polysorbate 80, cell culture media, and trace amounts of fetal bovine serum. RotaTeq contains no preservatives. RotaTeq is a pale yellow clear liquid that may have a pink tint. The plastic dosing tube and cap are not made with natural rubber latex.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
23/01/2011
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
רישום
136 76 31569 00
מחיר
0 ₪
מידע נוסף
