Quest for the right Drug
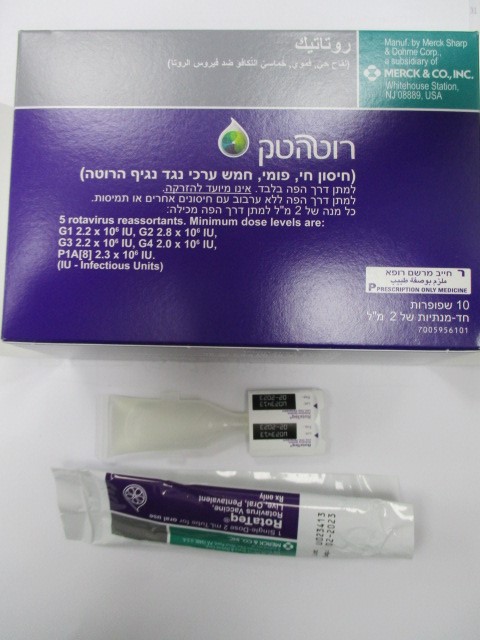
רוטטק ROTATEQ (ROTAVIRUS G1 REASSORTANT, ROTAVIRUS G2 REASSORTANT, ROTAVIRUS G3 REASSORTANT, ROTAVIRUS G4 REASSORTANT, ROTAVIRUS P1A[8] REASSORTANT)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
אין פרטים : ORAL SOLUTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
5 DOSAGE AND ADMINISTRATION FOR ORAL USE ONLY. NOT FOR INJECTION. The vaccination series consists of three ready-to-use liquid doses of RotaTeq administered orally starting at 6 to 12 weeks of age, with the subsequent doses administered at 4- to 10-week intervals. The third dose should not be given after 32 weeks of age [see Clinical Studies (16)]. There are no restrictions on the infant’s consumption of food or liquid, including breast milk, either before or after vaccination with RotaTeq. Do not mix the RotaTeq vaccine with any other vaccines or solutions. Do not reconstitute or dilute [see Dosage and Administration (5.2)]. For storage instructions [see How Supplied/Storage and Handling (17.1)]. Each dose is supplied in a container consisting of a squeezable plastic dosing tube with a twist-off cap, allowing for direct oral administration. The dosing tube is contained in a pouch [see Dosage and Administration (5.2)]. 5.1 Use with Other Vaccines In clinical trials, RotaTeq was administered concomitantly with other licensed pediatric vaccines [see Adverse Reactions (9.1), Drug Interactions (10.1), and Clinical Studies (16)]. 5.2 Instructions for Use To administer the vaccine: Tear open the pouch and remove the dosing tube. Clear the fluid from the dispensing tip by holding tube vertically and tapping cap. Open the dosing tube in 2 easy motions: 1. Puncture the dispensing tip by screwing cap clockwise until it becomes tight. 2. Remove cap by turning it counterclockwise. Administer dose by gently squeezing liquid into infant's mouth toward the inner cheek until dosing tube is empty. (A residual drop may remain in the tip of the tube.) If for any reason an incomplete dose is administered (e.g., infant spits or regurgitates the vaccine), a replacement dose is not recommended, since such dosing was not studied in the clinical trials. The infant should continue to receive any remaining doses in the recommended series. Discard the empty tube and cap in approved biological waste containers according to local regulations. 6 DOSAGE FORMS AND STRENGTHS RotaTeq, 2 mL for oral use, is a ready-to-use solution of live reassortant rotaviruses, containing G1, G2, G3, G4 and P1A[8] which contains a minimum of 2.0 – 2.8 x 106 infectious units (IU) per individual reassortant dose, depending on the reassortant, and not greater than 116 x 106 IU per aggregate dose. Each dose is supplied in a container consisting of a squeezable plastic dosing tube with a twist-off cap, allowing for direct oral administration. The dosing tube is contained in a pouch.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
23/01/2011
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
רישום
136 76 31569 00
מחיר
0 ₪
מידע נוסף
