Quest for the right Drug
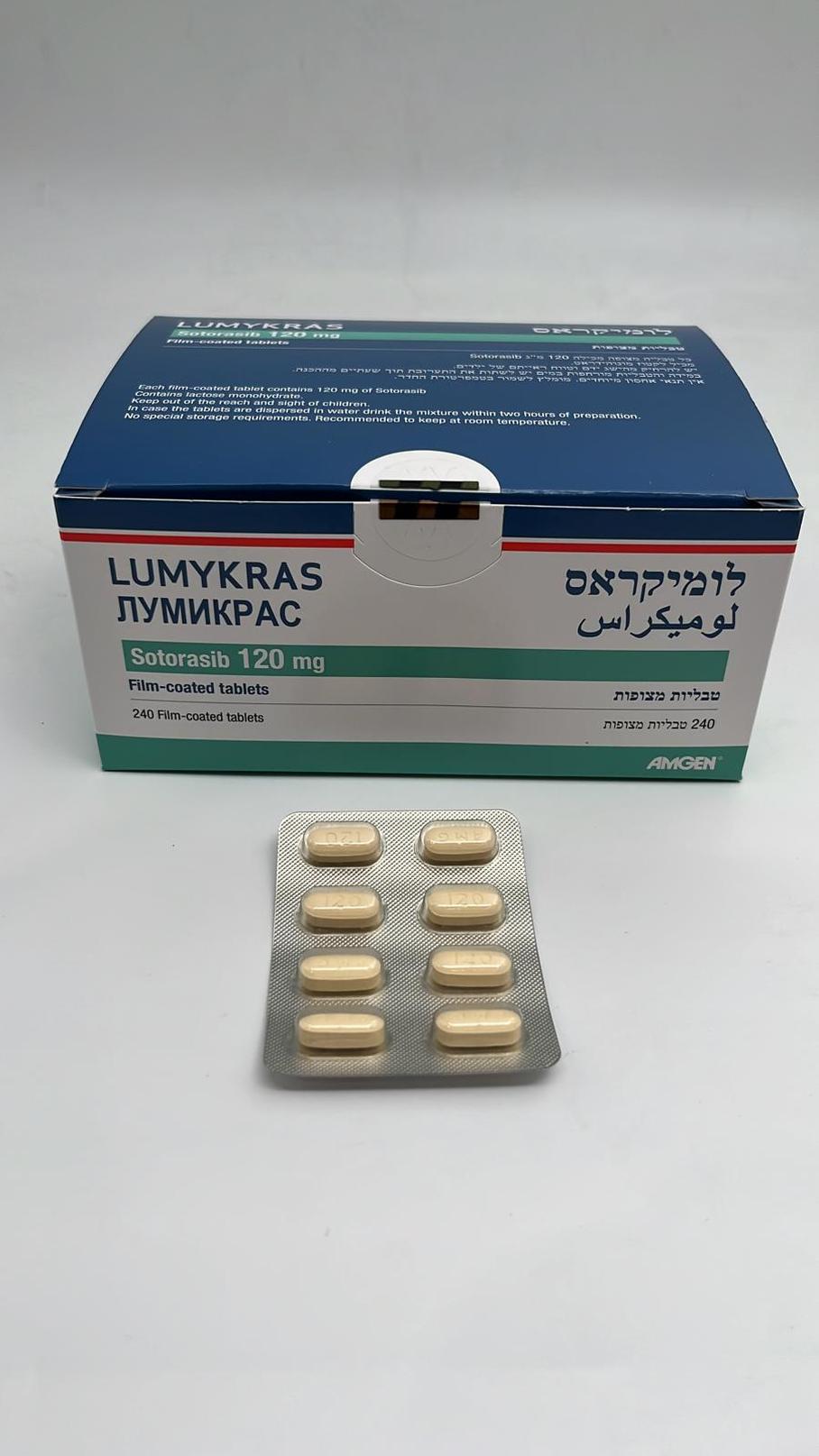
לומיקראס LUMYKRAS (SOTORASIB)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות מצופות פילם : FILM COATED TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Hepatotoxicity Sotorasib can cause hepatotoxicity, which may lead to drug-induced liver injury (DILI) and hepatitis. Sotorasib has been associated with transient elevations of serum transaminases (ALT and AST). These elevations improved or resolved with dose modification or permanent discontinuation of treatment and did not result in any cases of liver failure or fatal cases in clinical studies. Among patients who experienced hepatotoxicity, 38% had hepatotoxicity leading to dose interruption or dose reduction. Overall, 26% of patients with hepatotoxicity received concurrent corticosteroids. Cases of liver enzyme increase can be asymptomatic. Patients should be monitored for liver function (ALT, AST, and total bilirubin) prior to the start of LUMYKRAS, every 3 weeks for the first 3 months of treatment, then once a month or as clinically indicated, with more frequent testing in patients who develop transaminase and/or bilirubin elevations. Based on the severity of the laboratory abnormalities, treatment with LUMYKRAS must be stopped until recovered to ≤ grade 1 or to baseline grade, and the dose must either be modified or permanently discontinue treatment as recommended (see section 4.2). Interstitial Lung Disease (ILD)/pneumonitis ILD/pneumonitis occurred in patients treated with LUMYKRAS with prior exposure to immunotherapy or radiotherapy (see section 4.8). Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (e.g. dyspnoea, cough, fever). Immediately withhold LUMYKRAS in patients with suspected ILD/pneumonitis and permanently discontinue LUMYKRAS if no other potential causes of ILD/pneumonitis are identified (see section 4.2). Lactose intolerance LUMYKRAS contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Sodium This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
Effects on Driving
4.7 Effects on ability to drive and use machines LUMYKRAS has no or negligible influence on the ability to drive and use machines.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
