Quest for the right Drug
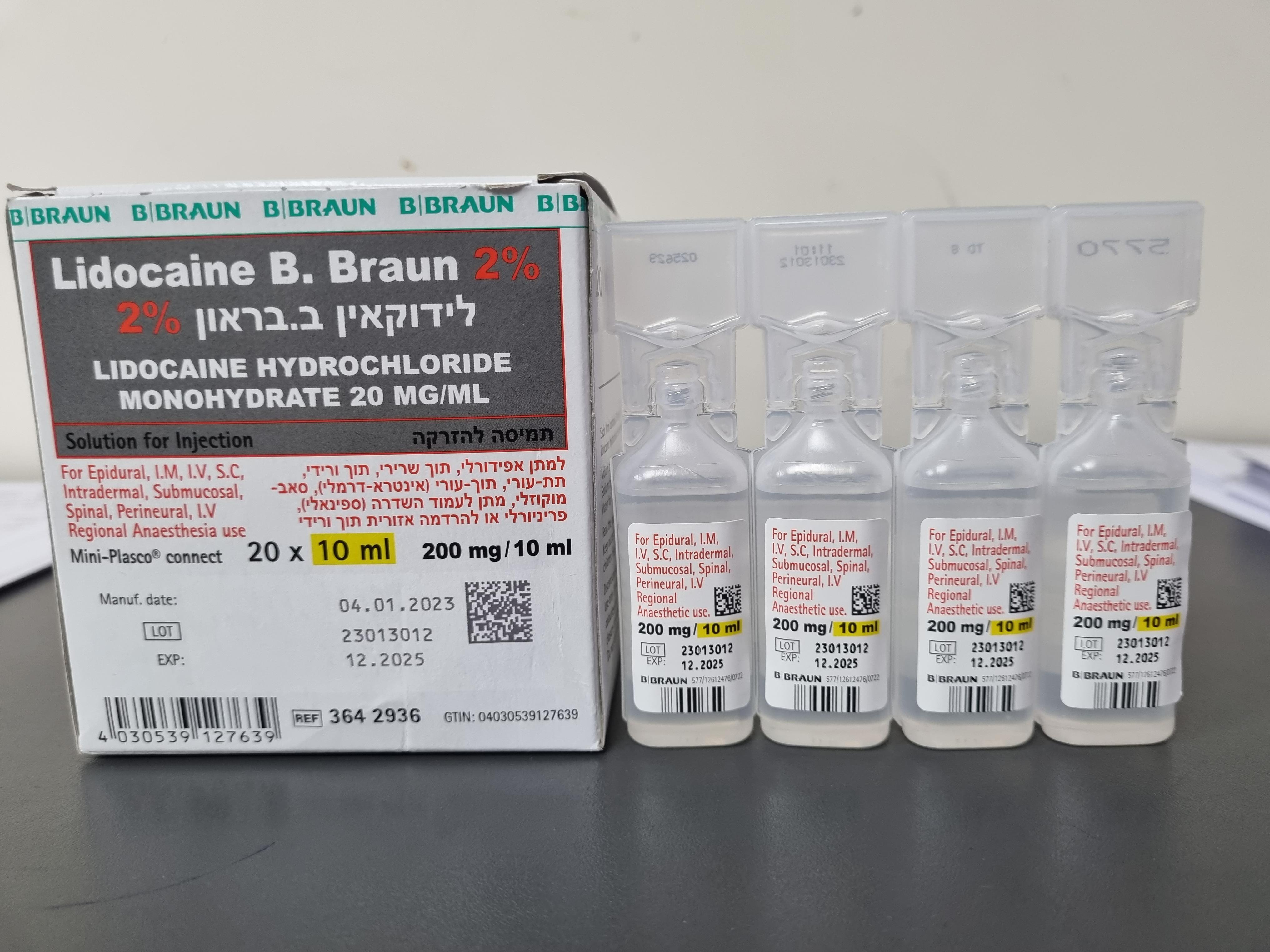
לידוקאין ב.בראון 2% LIDOCAINE B.BRAUN 2% (LIDOCAINE HYDROCHLORIDE MONOHYDRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי, תוך-שרירי, אפידורל, תת-עורי, תוך-עורי, הזרקה תת-רירית, שדרתי, לסביבת העצב, : I.V, I.M, EPIDURAL, S.C, INTRADERMAL, SUBMUCOSAL, SPINAL, PERINEURAL, I.V REGIONAL ANAESTHESIA
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Overdose : מינון יתר
4.9 Overdose The toxic effects of lidocaine depend on the level of the plasma concentration; the higher the plasma concentration and the more rapid its rise, the more frequent and more serious are the toxic reactions. Depending on the individual sensitivity, toxic reactions occur from a concentration of approximately 5 ‑ 9 mg lidocaine per litre upward in venous blood. The lethal plasma concentration for humans is in the range 6 to 33 mg lidocaine per litre. Symptoms Effects on the CNS: Low toxic overdoses of lidocaine result in stimulation of the CNS. Gross overdose, producing high toxic plasma concentrations, causes depression of the central functions. Two phases of lidocaine intoxication can be distinguished: Stimulation At the beginning of intoxication with lidocaine patients mainly show symptoms of excitation: unrest, vertigo, disturbances of hearing and vision, unpleasant perioral sensations, agitation, hallucination, euphoria, paraesthesias (e.g. circumoral paraesthesia and numbness of the tongue), dizziness, tinnitus, blurred visions, nausea, vomiting, dysarthria. Shivering and muscular twitching may be signs of imminent attacks of generalized convulsion. Subconvulsive plasma levels of lidocaine often also lead to sleepiness and sedation. Tachycardia, hypertension and flushing may occur as a sign of initial stimulation of the sympathetic nervous system. Depression During progress of the intoxication of the CNS increasing impairment of the brain stem functions appears in the form of respiratory depression and coma, even up to death. Effects on cardiovascular circulation: Unpalpable pulse, pallor, hypotension, bradycardia, arrhythmias, cardiovascular collapse, ventricular fibrillation, cardiac arrest. Sudden hypotension often is the first sign of cardiovascular toxicity of lidocaine. The hypotension is mainly caused by the reduction or block of cardiac impulse conduction. These toxic effects, however, are less relevant than those on the CNS. Treatment The occurrence of central nervous or cardiovascular symptoms demands the following emergency treatment: • Immediately discontinue administration. • Ensure patency of the airways. • Supply additional oxygen. If necessary provide artificial ventilation with pure oxygen – assisted or controlled – initially via mask and air bag, then intubate. The oxygen therapy must be continued until all vital functions have returned to normal. • Monitor blood pressure, pulse and pupil width carefully. • Maintain the circulation by sufficient supply of intravenous fluid. • Immediately start cardio-pulmonary resuscitation, if necessary. These measures are also applicable in the case of accidental total spinal anaesthesia, first manifesting as unrest, whispering voice, and sleepiness. The latter can proceed to unconsciousness and respiratory arrest. Further therapeutic measures include the following: Acute life-threatening hypotension should be treated with intravenous vasopressors. Bradycardia caused by increased vagal tone should be treated with intravenous atropine. Convulsions not reacting to sufficient oxygenation should be treated with intravenous benzodiazepins or ultra-short-acting barbiturates. Centrally acting analeptics are contra-indicated. There is no specific antidote. Lidocaine cannot be eliminated by haemodialysis.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
