Quest for the right Drug
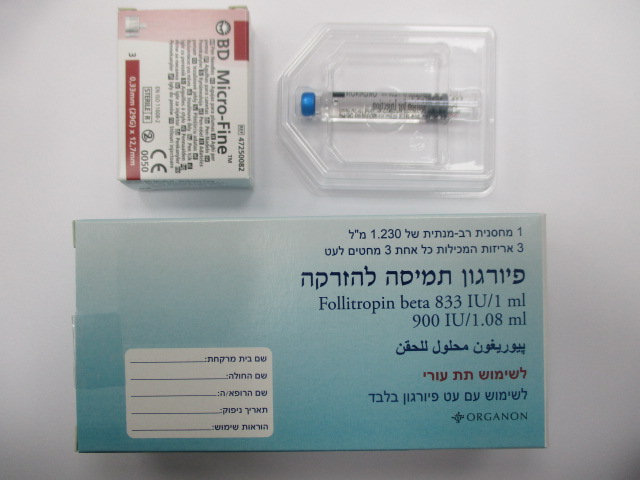
פיורגון תמיסה להזרקה PUREGON SOLUTION FOR INJECTION (FOLLITROPIN BETA)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Clinical use of Puregon by the intramuscular or subcutaneous routes may lead to local reactions at the site of injection (3% of all patients treated). The majority of these local reactions are mild and transient in nature. Generalised hypersensitivity reactions have been observed uncommonly (approximately 0.2% of all patients treated with follitropin beta). Cases of anaphylactic reactions (including those requiring hospitalisation) have been reported in the post-marketing setting. Treatment of females: In approximately 4% of the women treated with follitropin beta in clinical trials, signs and symptoms related to ovarian hyperstimulation syndrome (OHSS) have been reported (see section 4.4). Adverse reactions related to this syndrome include pelvic pain and/or congestion, abdominal pain and/or distension, breast complaints and ovarian enlargement. The table below lists the adverse reactions with follitropin beta reported in clinical trials and post-marketing surveillance in females, according to system organ class and frequency; common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100) and not known (cannot be estimated from available data). SOC Frequency Adverse reaction Immune system disorders Not known Anaphylactic reactions Nervous system disorders Common Headache Gastrointestinal disorders Common Abdominal distension Abdominal pain Uncommon Abdominal discomfort Constipation Diarrhoea Nausea Reproductive system and Common OHSS breast disorders Pelvic pain Uncommon Breast complaints1 Metrorrhagia Ovarian cyst Ovarian enlargement Ovarian torsion Uterine enlargement Vaginal haemorrhage General disorders and Common Injection site reaction2 administration site conditions Uncommon Generalised hypersensitivity reaction3 1. Breast complaints include tenderness, pain and/or engorgement and nipple pain 2. Local reactions at the site of injection include: bruising, pain, redness, swelling and itching 3. Generalised hypersensitivity reaction include erythema, urticaria, rash and pruritus In addition, ectopic pregnancy, miscarriage and multiple gestations have been reported. These are considered to be related to ART or subsequent pregnancy. In rare instances, thromboembolism has been associated with follitropin beta / hCG therapy as with other gonadotrophins. Treatment of males: The table below lists the adverse reactions with follitropin beta reported in a clinical trial in males (30 patients dosed) and post-marketing surveillance, according to system organ class and frequency; common (≥ 1/100 to < 1/10) and not known (cannot be estimated from available data). SOC Frequency1 Adverse reaction Immune system disorders Not known Anaphylactic reactions Nervous system disorders Common Headache Skin and subcutaneous Common Acne tissue disorders Rash Reproductive system and Common Epididymal cyst breast disorders Gynaecomastia General disorders and Common Injection site reaction2 administration site conditions 1. Adverse reactions that are reported only once are listed as common because a single report raises the frequency above 1%. 2. Local reactions at the site of injection include induration and pain. Side effects can be reported to the Ministry of Health by using the online form for reporting side effects in the homepage of the ministry of health website www.health.gov.il or by entering the following link: https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2002
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
04.04.21 - עלון לצרכן אנגלית 04.04.21 - עלון לצרכן עברית 26.07.21 - עלון לצרכן ערבית 08.11.22 - עלון לצרכן אנגלית 08.11.22 - עלון לצרכן עברית 08.11.22 - עלון לצרכן ערבית 04.12.23 - עלון לצרכן עברית 18.01.24 - עלון לצרכן אנגלית 18.01.24 - עלון לצרכן ערבית 11.04.24 - עלון לצרכן עברית 21.05.24 - עלון לצרכן אנגלית 21.05.24 - עלון לצרכן ערבית 13.08.15 - החמרה לעלון 10.06.20 - החמרה לעלון 04.04.21 - החמרה לעלון 08.11.22 - החמרה לעלון 29.03.24 - החמרה לעלון 11.04.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
פיורגון תמיסה להזרקה