Quest for the right Drug
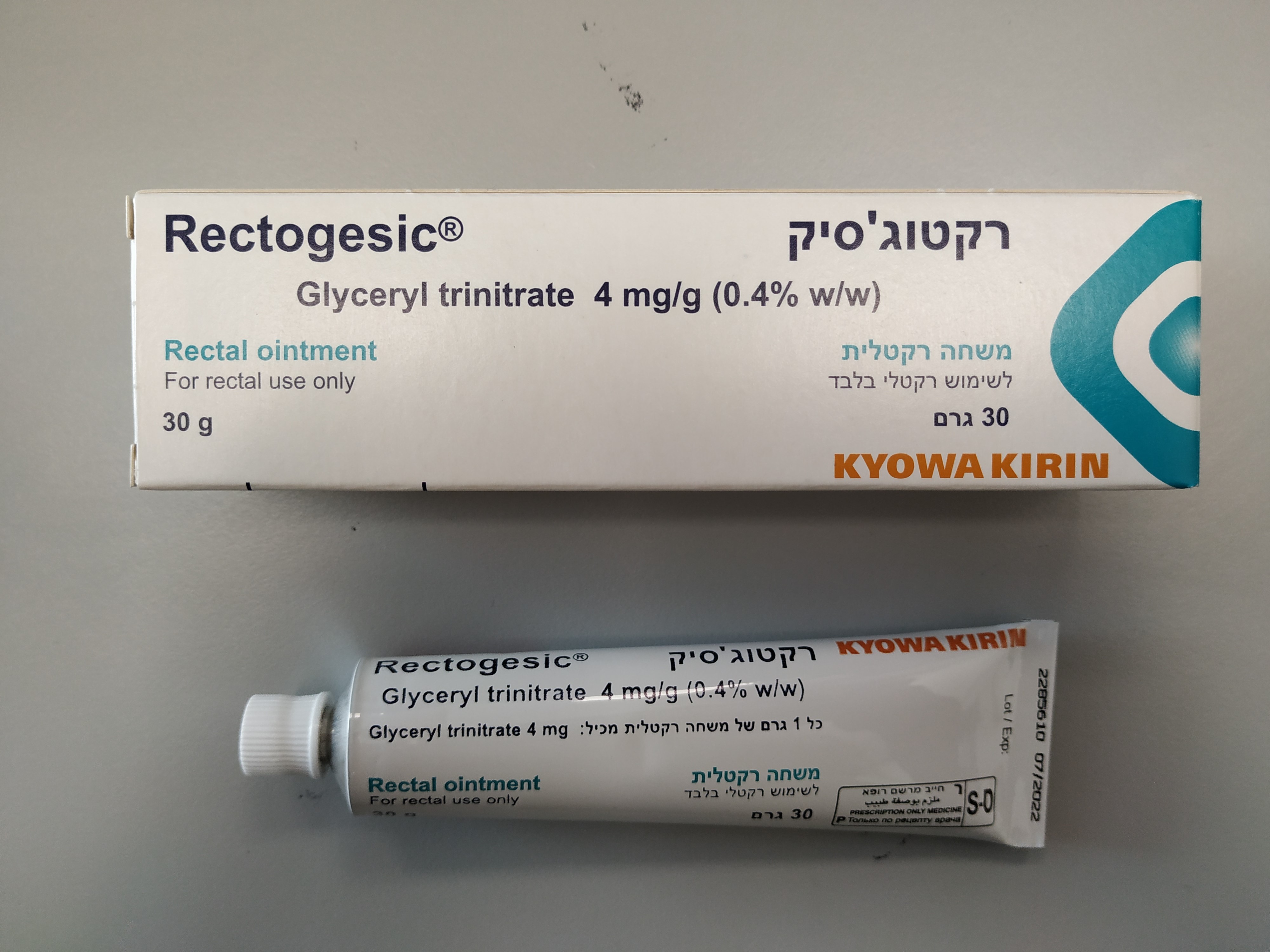
רקטוג'סיק ® RECTOGESIC ® (GLYCERYL TRINITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
רקטלי : RECTAL
צורת מינון:
משחה : OINTMENT
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects In patients treated with Rectogesic 4 mg/g Rectal Ointment, the most common treatment related adverse reaction was dose-related headache which occurred with an incidence of 57%. Adverse reactions from clinical studies are displayed by system organ class in the table below. Within the system organ class, the adverse reactions are listed by frequency using the following groupings: very common (> 1/10), common (>1/100 <1/10), uncommon (>1/1000 <1/100). System Organ Class Frequency Adverse Reaction Nervous system disorder Very common Headache Common Dizziness Gastrointestinal disorders Common Nausea Uncommon Diarrhoea, anal discomfort, vomiting, rectal bleeding, rectal disorder Skin and subcutaneous tissue Uncommon Pruritus, anal burning and disorders itching Cardiovascular system Uncommon Tachycardia disorders Adverse reactions to glyceryl trinitrate are generally dose-related and almost all of these reactions are the result of vasodilator activity. Headache, which may be severe, is the most commonly reported side effect. In the Phase III clinical trials with Rectogesic 4 mg/g Rectal Ointment the incidence of mild, moderate and severe headache was 18%, 25% and 20%. Patients with a previous history of migraine or recurrent headache were at a higher risk of developing headache during treatment (see Section 4.3). Headache may be recurrent with each daily dose, especially at higher doses. Headache can be treated with mild analgesics e.g. paracetamol and is reversible on discontinuation of treatment. Rare cases of orthostatic hypotension-type events associated with symptoms of vertigo and dizziness were reported in clinical trials. There was no discernible dose-related trend in the incidence of these events. The orthostatic hypotension-type event was of mild intensity in the majority of these patients, and there were no severe orthostatic hypotension-type events reported during the Phase III clinical studies. Dizziness and vertigo contributed to the discontinuation of glyceryl trinitrate in a few cases. Post-marketing Experience Because these reactions are received from spontaneous reporting, the frequency is not known (cannot be estimated from the available data). Nervous system disorders: Lightheadedness, syncope Vascular disorders: Hypotension, orthostatic hypotension Immune system disorders: Hypersensitivity, anaphylactoid reaction General disorders and administration site conditions: Application site irritation, application site rash, application site pain Lightheadedness and hypotension (including orthostatic hypotension) in some patients may be severe enough to warrant discontinuation of therapy. Class effects Extremely rarely, ordinary doses of organic nitrates have caused methaemoglobinaemia in normal–seeming patients. Flushing, unstable angina and withdrawal hypertension may also occur. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form http://forms.gov.il/globaldata/getsequence/getsequence.aspx?formType=AdversEffectMedic@ moh.gov.il In additionally, you can report to Perrigo via the following address: www.perrigo-pharma.co.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
