Quest for the right Drug
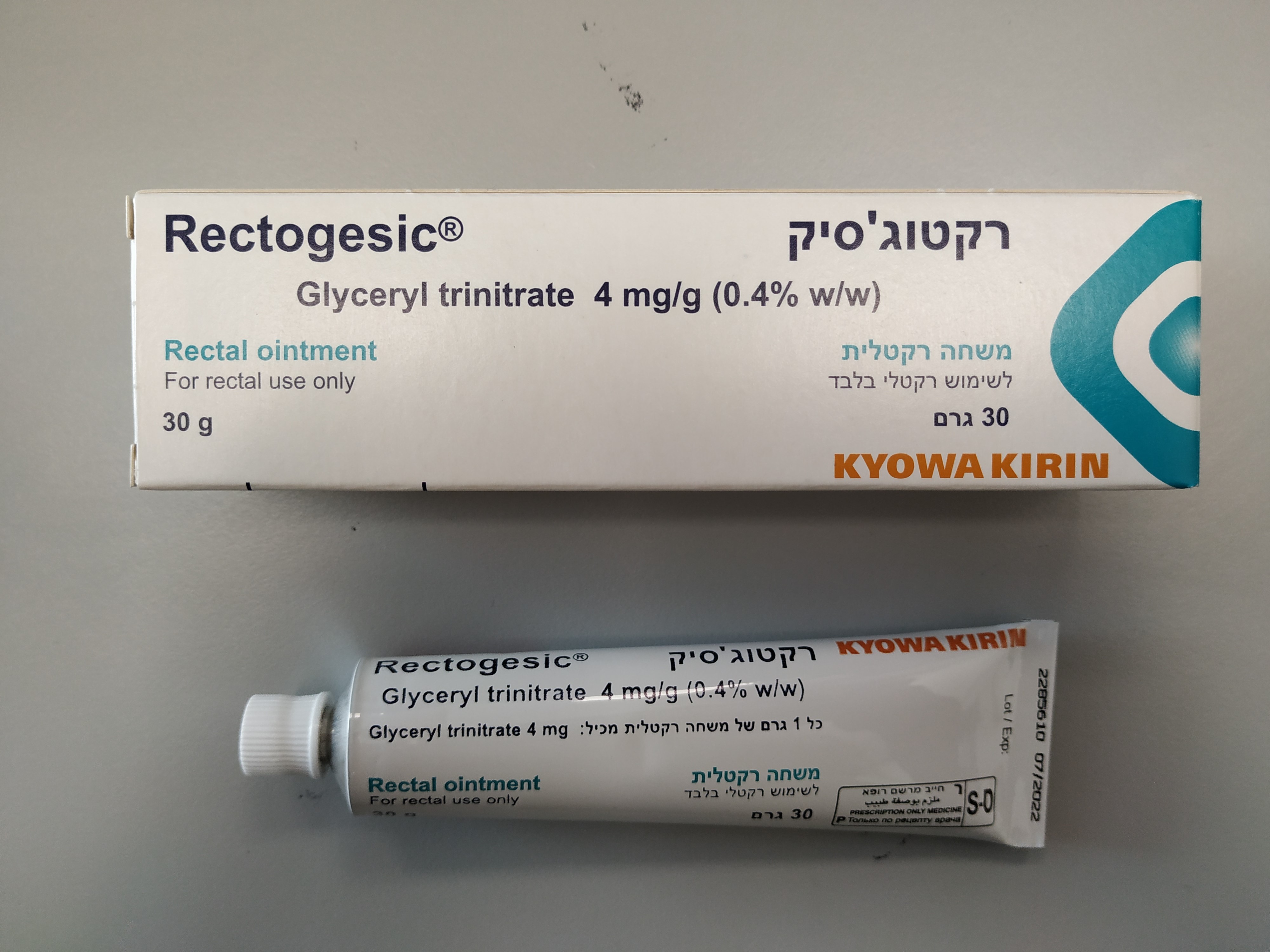
רקטוג'סיק ® RECTOGESIC ® (GLYCERYL TRINITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
רקטלי : RECTAL
צורת מינון:
משחה : OINTMENT
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Route of administration: rectal use Adults: A finger covering, such as cling film or a finger cot, may be placed on the finger to be used to apply the ointment. (Finger cots to be obtained separately from local pharmacy or surgical supplies retailer or cling film from local store.) The finger is placed along side a 2.5cm dosing line which is provided on the outside carton in which Rectogesic is supplied, and a strip of ointment the length of the line is expressed onto the end of the finger by gently squeezing the tube. The amount of ointment expressed is approximately 375 mg (1.5 mg GTN). The covered finger is then gently inserted into the anal canal to the distal interphalangeal joint of the finger and applied circumferentially to the anal canal. The dose delivered from the 4 mg/g ointment is 1.5 mg glyceryl trinitrate. The dose is to be applied intra-anally every twelve hours. Treatment may be continued until the pain abates, up to a maximum of 8 weeks. Rectogesic should be used following conservative treatment failure for acute symptoms of anal fissure. Elderly (over 65 years): No specific information concerning the usage of Rectogesic in the elderly is available. Patients with Hepatic or Renal Impairment No specific information concerning the usage of Rectogesic in patients with hepatic or renal impairment is available. Children and Adolescents: Rectogesic is not recommended for use in children and adolescents below 18 years of age due to a lack of data on safety and efficacy.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
